ABTCytochrome P450 inhibitor; inhibits 20-HETE synthesis CAS# 1614-12-6 |
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- BYK 49187
Catalog No.:BCC2450
CAS No.:163120-31-8
- A-966492
Catalog No.:BCC2211
CAS No.:934162-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1614-12-6 | SDF | Download SDF |
| PubChem ID | 1367 | Appearance | Powder |
| Formula | C6H6N4 | M.Wt | 134.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (931.86 mM; Need ultrasonic) H2O : 50 mg/mL (372.74 mM; Need ultrasonic) | ||
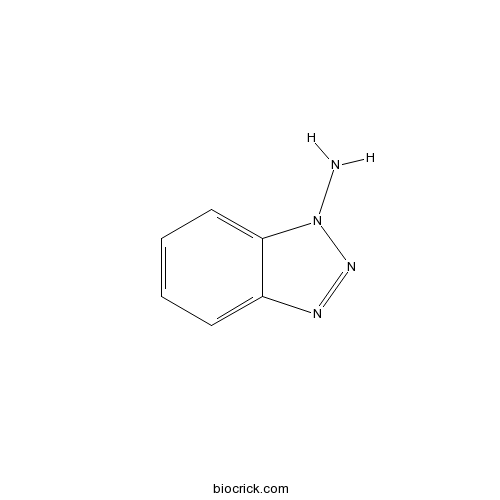
| Chemical Name | benzotriazol-1-amine | ||
| SMILES | C1=CC=C2C(=C1)N=NN2N | ||
| Standard InChIKey | JCXKHYLLVKZPKE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H6N4/c7-10-6-4-2-1-3-5(6)8-9-10/h1-4H,7H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cytochrome P450 inhibitor. Inhibits synthesis of the eicosanoid 20-hydroxyeicosatetraenoic acid (20-HETE). Reduces intimal hyperplasia and vascular remodeling following endothelial injury in rat carotid arteries. |

ABT Dilution Calculator

ABT Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.4549 mL | 37.2745 mL | 74.549 mL | 149.098 mL | 186.3724 mL |
| 5 mM | 1.491 mL | 7.4549 mL | 14.9098 mL | 29.8196 mL | 37.2745 mL |
| 10 mM | 0.7455 mL | 3.7274 mL | 7.4549 mL | 14.9098 mL | 18.6372 mL |
| 50 mM | 0.1491 mL | 0.7455 mL | 1.491 mL | 2.982 mL | 3.7274 mL |
| 100 mM | 0.0745 mL | 0.3727 mL | 0.7455 mL | 1.491 mL | 1.8637 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- Fmoc-Chg-OH
Catalog No.:BCC3164
CAS No.:161321-36-4
- 1-O-Acetyl-6-O-isobutyrylbritannilactone
Catalog No.:BCN7795
CAS No.:1613152-34-3
- SR-9243
Catalog No.:BCC3983
CAS No.:1613028-81-1
- H-Orn(Z)-OtBu.HCl
Catalog No.:BCC2677
CAS No.:161234-80-6
- Cimicidanol 3-O-alpha-L-arabinoside
Catalog No.:BCN6528
CAS No.:161207-05-2
- H-Asp(OEt)-OEt.HCl
Catalog No.:BCC2888
CAS No.:16115-68-7
- Bidwillol A
Catalog No.:BCN4858
CAS No.:161099-42-9
- Cimicifugoside H2
Catalog No.:BCN7949
CAS No.:161097-77-4
- Formyl-DL-Trp-OH
Catalog No.:BCC3120
CAS No.:16108-03-5
- Epimedokoreanin C
Catalog No.:BCN8080
CAS No.:161068-54-8
- SIN-1 chloride
Catalog No.:BCC5670
CAS No.:16142-27-1
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
ABT-737 Synergizes with Cisplatin Bypassing Aberration of Apoptotic Pathway in Non-small Cell Lung Cancer.[Pubmed:28319809]
Neoplasia. 2017 Apr;19(4):354-363.
A subset of non-small cell lung cancer (NSCLC), which does not have a druggable driver mutation, is treated with platinum-based cytotoxic chemotherapy, but it develops resistance triggered by DNA damage responses. Here, we investigated the effect of activation of STAT3 by cisplatin on anti-apoptotic proteins and the effectiveness of a co-treatment with cisplatin and a BH3 mimetic, ABT-737. We analyzed the relationship between cisplatin and STAT3 pathway and effect of ABT-737, when combined with cisplatin in NSCLC cells and K-ras mutant mouse models. The synergism of this combination was evaluated by the Chou-Talalay Combination Index method. In vivo activity was evaluated by micro-CT. In NSCLC cells, there was a time and dose-dependent phosphorylation of SRC-JAK2-STAT3 by cisplatin, followed by increased expression of anti-apoptotic molecules. When the expression of the BCL-2 protein family members was evaluated in clinical samples, BCL-xL was most frequently overexpressed. Dominant negative STAT3 suppressed their expression, suggesting that STAT3 mediates cisplatin mediated overexpression of the anti-apoptotic molecules. ABT-737 displaced BCL-xL from mitochondria and induced oligomerization of BAK. ABT-737 itself showed cytotoxic effects and a combination of ABT-737 with cisplatin showed strong synergistic cytotoxicity. In a murine lung cancer model, co-treatment with ABT-737 and cisplatin induced significant tumor regression. These findings reveal a synergistic cytotoxic and anti-tumor activity of ABT-737 and cisplatin co-treatment in preclinical models, and suggest that clinical trials using this strategy may be beneficial in advanced NSCLC.
B-cell lymphoma 2 inhibitor ABT-737 induces Beclin1- and reactive oxygen species-dependent autophagy in Adriamycin-resistant human hepatocellular carcinoma cells.[Pubmed:28351336]
Tumour Biol. 2017 Mar;39(3):1010428317695965.
ABT-737, a B-cell lymphoma 2 homology 3 mimetic, not only induces cell apoptosis by inhibiting the interaction of B-cell lymphoma 2 and Bax but also induces cell autophagy by interrupting the interaction of B-cell lymphoma 2 and Beclin1. Several recent studies have reported that ABT-737 has antitumor efficacy in diverse cancers. However, another study showed that hepatocellular carcinoma cells with high B-cell lymphoma 2 expression were resistant to ABT-737 compared to hepatocellular carcinoma cells with low B-cell lymphoma 2 expression. It was also found that ABT-737-induced autophagy is crucial for drug resistance. Here, we observed that of B-cell lymphoma 2 expression in Adriamycin-resistant human hepatocellular carcinoma HepG2/ADM cells is higher than that in human hepatocellular carcinoma HepG2 cells. Therefore, we further confirmed the mechanism and effect of autophagy induced by ABT-737 on apoptosis in HepG2/ADM cells with high B-cell lymphoma 2 expression. Our results showed that ABT-737 induced apoptosis and autophagy in time- and dose-dependent manner in HepG2/ADM cells, and this ABT-737-induced autophagy was Beclin1-dependent. In addition, we demonstrated that ABT-737 induced reactive oxygen species-mediated autophagy, and the reactive oxygen species-inhibitor N-acetyl-l-cysteine suppressed the reactive oxygen species-induced autophagy and ABT-737-induced increase in HepG2/ADM cell apoptosis. Furthermore, autophagy inhibitors increased HepG2/ADM cell apoptosis. In conclusion, our study further confirms that Beclin1- and reactive oxygen species-dependent autophagy induced by ABT-737 also plays a protective function in HepG2/ADM cells, which show B-cell lymphoma 2 expression higher than that in HepG2 cells.
Combination of ABT-737 and resveratrol enhances DNA damage and apoptosis in human T-cell acute lymphoblastic leukemia MOLT-4 cells.[Pubmed:28366708]
Toxicol In Vitro. 2017 Aug;42:38-46.
ABT-737 belongs to a new class of anticancer agents named BH3 mimetics. ABT-737 competitively binds to surface hydrophobic grooves of anti-apoptotic proteins of Bcl-2 family, counteracting their protective effect. Resveratrol is a natural polyphenol that has been shown to inhibit the proliferation and/or induce apoptosis in a number of different types of cancer cells. The present study was designed to analyze the combined effects of ABT-737 and resveratrol on human acute lymphoblastic leukemia cells. The in vitro cytotoxic activity of these agents against MOLT-4 leukemia cells was determined using the Coulter electrical impedance method, comet assay, and flow cytometry, light microscopy and western blot techniques. The results are the first data showing that ABT-737 combined with resveratrol markedly decreased the cell viability, increased DNA damage, caused the cell cycle perturbation, and synergistically enhanced apoptosis in MOLT-4 cells, when compared to the data obtained after application of the single agent. Moreover, the simultaneous treatment of leukemia cells with ABT-737 and resveratrol resulted in a reduction in mitochondrial membrane potential, an increase of p53 protein level and up-regulation of the Bax/Bcl-2 ratio. The obtained data indicate that the combination of ABT-737 and resveratrol is a promising approach for acute lymphoblastic leukemia treatment that should be further explored.
Evaluation of the effect of the EGFR antibody-drug conjugate ABT-414 on QT interval prolongation in patients with advanced solid tumors likely to over-express EGFR.[Pubmed:28349167]
Cancer Chemother Pharmacol. 2017 May;79(5):915-922.
PURPOSE: ABT-414 is an antibody-drug conjugate (ADC) being developed for the treatment of tumors harboring amplification of the epidermal growth factor receptor (EGFR). This study evaluated the potential of ABT-414 to prolong the QT interval as part of the initial phase 1 study (NCT01741727). METHODS: Data from patients who received ABT-414 monotherapy at a dose of 1-4 mg/kg once every 3 weeks or 1 or 1.5 mg/kg weekly for 2 out of every 3 weeks (alternate schedule) by intravenous infusion were included in the analysis of triplicate 12-lead ECGs obtained before dosing and through 168 h after dosing. Data from time-matched pharmacokinetic samples and QT interval assessments were evaluated using linear mixed-effects modeling to determine the effects of ABT-414, total ABT-806, and cysteine-maleimidocaproyl monomethyl auristatin F (Cys-mcMMAF) on the QT interval corrected using Fridericia's formula (QTcF). RESULTS: Fifty-one patients were included in the analyses. ABT-414 had no clinically meaningful effect on QTcF. Using pooled data from doses >/=2 mg/kg, the estimated mean QTcF reached a maximum of 4.30 ms after dosing, with a one-sided 95% upper confidence bound of 8.32 ms. The exposure-response analysis showed no statistically significant relationship between DeltaQTcF and the concentration of any analyte (P > 0.05). No patient had a QTcF value >480 ms or a QTcF value >30 ms. CONCLUSIONS: ABT-414 had no clinically meaningful effect on the QTcF interval at doses being evaluated for treatment of patients with solid tumors.
20-Hydroxyeicosatetraenoic acid inhibition attenuates balloon injury-induced neointima formation and vascular remodeling in rat carotid arteries.[Pubmed:23658377]
J Pharmacol Exp Ther. 2013 Jul;346(1):67-74.
20-Hydroxyeicosatetraenoic acid (20-HETE) contributes to the migration and proliferation of vascular smooth muscle cells (VSMC) in vitro, but there are few studies that address its effects on vascular remodeling in vivo. The present study determined whether inhibition of 20-HETE production attenuates intimal hyperplasia (IH) and vascular remodeling after balloon injury (BI). Sprague Dawley rats underwent BI of the common carotid artery and were treated with vehicle, 1-aminobenzotriazole (ABT, 50 mg/kg i.p. once daily), or HET0016 (N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine) (2 mg/kg s.c. twice daily) for 14 days. Fourteen days after BI and treatment, the animals underwent carotid angiography, and the arteries were harvested for morphometric, enzymatic and immunohistochemical analysis. There was a 96% reduction of angiographic stenosis in the rats treated with 1-ABT. There was a 61 and 66% reduction of the intima/media area ratios in the 1-ABT and HET0016 treated rats compared with the vehicle-treated group. 20-HETE levels were elevated in BI carotid arteries, and the levels were markedly suppressed in the groups treated with 1-ABT and HET0016 (P < 0.001). Immunostaining revealed that the expression of CYP4A enzyme was markedly increased in the neointima of BI arteries, and it colocalized with the expression of smooth muscle-specific actin, indicating increased proliferation of VSMC. An increase in the expression of CYP4A and the production of 20-HETE contributes to neointimal growth in BI rat carotid arteries. Systemic administration 1-ABT or HET0016 prevents the increase in 20-HETE levels and attenuates VSMC migration and proliferation, resulting in a marked reduction in IH and vascular remodeling after endothelial injury.
Inactivation of rabbit pulmonary cytochrome P-450 in microsomes and isolated perfused lungs by the suicide substrate 1-aminobenzotriazole.[Pubmed:4045721]
J Pharmacol Exp Ther. 1985 Oct;235(1):186-90.
The autocatalytic destruction of pulmonary cytochrome P-450 (P-450) by 1-aminobenzotriazole (ABT) was investigated in microsomes and in isolated perfused lungs from untreated and beta-naphthoflavone-induced rabbits. Microsomal benzphetamine N-demethylase (BND) and 7-ethoxyresorufin O-deethylation (ERF) activities, catalyzed by P-450 isozymes 2 and 6, respectively, and specific P-450 content were determined after incubation with ABT. In vitro destruction of P-450 was dependent on ABT concentration and required NADPH. Significant losses of BND and ERF activities were observed only at ABT concentrations above 10 microM. Percent losses of BND and ERF activities equaled those of total P-450 at 1 mM and surpassed them at 10 mM. The time and concentration dependence of the destruction of P-450 by ABT was investigated in isolated perfused rabbit lungs. The percent loss of total P-450 increased with increasing ABT concentration (18 +/- 8% loss at 1 microM to 85 +/- 4% at 10 mM). Although extensive losses of P-450 occurred after perfusion with 10 mM ABT for 60 min, no ABT-dependent losses of flavin-containing monooxygenase activity were observed under these conditions. Percent losses of BND activity in these experiments were similar to those of total P-450 at 1 and 10 mM ABT but were less than P-450 losses at 0.01 and 0.1 mM ABT. Losses of ERF activity in lungs from beta-naphthoflavone-pretreated animals were also substantial and dependent upon perfusion time. Perfusion with 1 mM ABT for 2 to 60 min resulted in time-dependent losses of P-450 (42.8 +/- 7.2% at 2 min to 70.5 +/- 2.5% at 60 min) with equal or somewhat lesser diminishment of BND and ERF activities.(ABSTRACT TRUNCATED AT 250 WORDS)


