beta-Amyrin acetateCAS# 1616-93-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1616-93-9 | SDF | Download SDF |
| PubChem ID | 345510 | Appearance | Powder |
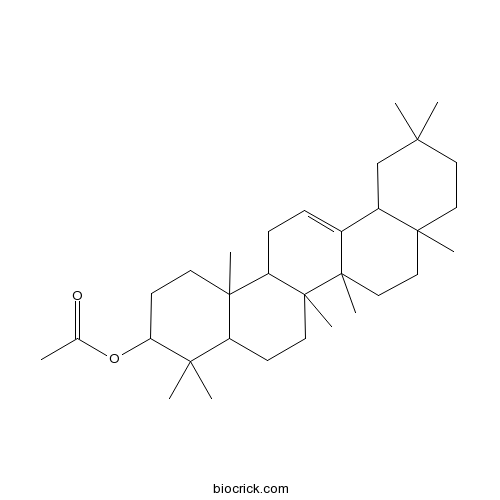
| Formula | C32H52O2 | M.Wt | 468.8 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4,4,6a,6b,8a,11,11,14b-octamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl) acetate | ||
| SMILES | CC(=O)OC1CCC2(C(C1(C)C)CCC3(C2CC=C4C3(CCC5(C4CC(CC5)(C)C)C)C)C)C | ||
| Standard InChIKey | UMRPOGLIBDXFNK-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. beta-Amyrin acetate and β-amyrin palmitate as antidyslipidemic agents, they show significant HMG-CoA-reductase inhibition. 2. beta-Amyrin acetate and honokiol exhibit sEH inhibitory activity. 3. beta-Amyrin acetate has a high antioxidant potential with an IC50 value of 158.2ug/mL, and has cytotoxicity , it could be a viable source of antioxidant and cytotoxic agents in cancer chemotherapy in the near future. 4. beta-Amyrin acetate has anti-inflammatory activity,with ID50 values in the range of 0.15-0.75 micromol/ear. 5. beta-Amyrin acetate exhibits weak-moderate antiproliferative activity against the A2780 human ovarian cancer cell line. |
| Targets | Immunology & Inflammation related |

beta-Amyrin acetate Dilution Calculator

beta-Amyrin acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1331 mL | 10.6655 mL | 21.3311 mL | 42.6621 mL | 53.3276 mL |
| 5 mM | 0.4266 mL | 2.1331 mL | 4.2662 mL | 8.5324 mL | 10.6655 mL |
| 10 mM | 0.2133 mL | 1.0666 mL | 2.1331 mL | 4.2662 mL | 5.3328 mL |
| 50 mM | 0.0427 mL | 0.2133 mL | 0.4266 mL | 0.8532 mL | 1.0666 mL |
| 100 mM | 0.0213 mL | 0.1067 mL | 0.2133 mL | 0.4266 mL | 0.5333 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- SIN-1 chloride
Catalog No.:BCC5670
CAS No.:16142-27-1
- ABT
Catalog No.:BCC7998
CAS No.:1614-12-6
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
- Hispidanin B
Catalog No.:BCN7394
CAS No.:1616080-84-2
- VRT-1353385
Catalog No.:BCC6433
CAS No.:1616113-45-1
- EPZ015666
Catalog No.:BCC5653
CAS No.:1616391-65-1
- Erythrinin D
Catalog No.:BCN6858
CAS No.:1616592-59-6
- 1,5-difluoro-3-methyl-2-nitrobenzene
Catalog No.:BCN6404
CAS No.:1616526-80-7
- Erythrinin G
Catalog No.:BCN6857
CAS No.:1616592-61-0
- Erythrinin H
Catalog No.:BCN6870
CAS No.:1616592-62-1
- ONC201
Catalog No.:BCC3989
CAS No.:1616632-77-9
- Dodovisone A
Catalog No.:BCN6839
CAS No.:1616683-50-1
- Dodovisone B
Catalog No.:BCN6867
CAS No.:1616683-51-2
Anti-inflammatory and chemopreventive effects of triterpene cinnamates and acetates from shea fat.[Pubmed:20484832]
J Oleo Sci. 2010;59(6):273-80.
Four triterpene acetates, alpha-amyrin acetate (1a), beta-Amyrin acetate (2a), lupeol acetate (3a), and butyrospermol acetate (4a), and four triterpene cinnamates, alpha-amyrin cinnamate (1c), beta-amyrin cinnamate (2c), lupeol cinnamate (3c), and butyrospermol cinnamate (4c), were isolated from the kernel fat (n-hexane extract) of the shea tree (Vitellaria paradoxa; Sapotaceae). Upon evaluation of these eight triterpene esters for inhibitory activity against 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation (1 microg/ear) in mice, all of the compounds tested exhibited marked anti-inflammatory activity, with ID50 values in the range of 0.15-0.75 micromol/ear, and among which compound 3c showed the highest activity with ID(50) of 0.15 micromol/ear. Compound 3c (10 mg/kg) further exhibited anti-inflammatory activity on rat hind paw edema induced by carrageenan, with the percentage of inflammation at 1, 3, and 5 h of 35.4, 41.5, and 45.5%, respectively. The eight triterpene esters were then evaluated for their inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) in Raji cells as a primary screening test for inhibitors of tumor promoters. All the compounds showed moderate inhibitory effects. Furthermore, compound 3c exhibited inhibitory effect on skin tumor promotion in an in vivo two-stage carcinogenesis test using 7,12-dimethylbenz [a] anthracene (DMBA) as an initiator and TPA as a promoter. The biological activities of triterpene acetate and cinnamate esters, together with the exceptionally high levels of these triterpenes in shea fat, indicate that shea nuts and shea fat (shea butter) constitute a significant source of anti-inflammatory and anti-tumor promoting compounds.
Discovery of soluble epoxide hydrolase inhibitors from natural products.[Pubmed:24309146]
Food Chem Toxicol. 2014 Feb;64:225-30.
With the goal of developing soluble epoxide hydrolase (sEH) inhibitors with novel chemical structures, the sEH inhibitory activities of 30 natural compounds were evaluated using both a fluorescent substrate, 3-phenyl-cyano(6-methoxy-2-naphthalenyl)methyl ester- 2-oxiraneacetic acid, and a physiological substrate, 14,15-epoxyeicosatrienoic acid. To evaluate the selectivity of sEH inhibition, the inhibition of microsomal epoxide hydrolase (mEH), which plays a critical role in detoxification of toxic epoxides, was determined using human liver microsomes. Honokiol and beta-Amyrin acetate, isolated from Magnolia officinalis and Acer mandshuricum, respectively, displayed strong inhibition of sEH activity, with respective IC50 values of 0.57 muM and 3.4 muM determined using the fluorescent substrate, and 1.7 muM and 6.1 muM determined using 14,15-epoxyeicosatrienoic acid. mEH activity was decreased to 49% or 61% of control activity by 25 muM honokiol or beta-Amyrin acetate, respectively. These results suggest that beta-Amyrin acetate and honokiol exhibit sEH inhibitory activity, although their sEH selectivity should be improved.
beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity.[Pubmed:25026352]
Pharm Biol. 2014 Nov;52(11):1478-86.
CONTEXT: Alstonia boonei De Wild (Apocyanaceae) is used in ethnomedicine for the management of malaria, ulcer, rhematic pain, toothache, and inflammatory disorders. OBJECTIVE: To investigate the anti-inflammatory potential of beta-amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei using animal models. MATERIALS AND METHODS: Chromatographic purification of the crude methanol extract led to the isolation and structure elucidation of beta-amyrin and alpha-amyrin acetate. Their anti-inflammatory activities were evaluated in rodents using egg albumen-induced paw edema and xylene-induced ear edema models. The gastric ulcerogenic, in vivo leucocyte migration, and RBC membrane stabilization tests were also investigated. RESULTS: alpha-Amyrin acetate at 100 mg/kg showed significant (p < 0.05) inhibition of egg albumen-induced paw edema with % inhibition of 40 at the 5th hour. Oral administration up to 100 mg/kg did not produce significant (p > 0.01) irritation of the gastric mucosa while significant (p < 0.01) ulceration was recorded for indomethacin at 40 mg/kg compared with the negative control. At 100 mug/mL, both beta-amyrin and alpha-amyrin acetate inhibited heat-induced hemolysis to as much 47.2 and 61.5%, respectively, while diclofenac sodium (100 mug/mL) evoked only 40.5% inhibition. Both compounds at 100 microg/ear produced significant (p < 0.01) inhibition of ear edema in mice by 39.4 and 55.5%, respectively. Also at 100 mg/kg (p.o.) alpha-amyrin acetate evoked 60.3% reduction in total leucocyte count and significant (p < 0.05) suppression (47.9%) of neutrophil infiltration. DISCUSSION AND CONCLUSION: This study generally provided evidence of profound anti-inflammatory activity of beta-amyrin and alpha-amyrin acetate isolated from the Alstonia boonei stem bark.
beta-Amyrin acetate and beta-amyrin palmitate as antidyslipidemic agents from Wrightia tomentosa leaves.[Pubmed:22541636]
Phytomedicine. 2012 Jun 15;19(8-9):682-5.
The ethanolic extract and fractions of Wrightia tomentosa Roem. & Schult (Apocynaceae) leaves were tested in vivo for their antidyslipidemic activity in high fat diet (HFD) induced dyslipidemic hamsters. Activity guided isolation resulted in identification of antidyslipidemic compounds beta-AA and beta-AP. Compounds beta-AA and beta-AP decrease the levels of LDL by 36% and 44%, and increase the HDL-C/TC ratio by 49% and 28%, respectively, at a dose of 10mg/kg. In addition, the isolated compounds beta-AA and beta-AP showed significant HMG-CoA-reductase inhibition, which was further established by docking studies.


