SGC-CBP30Inhibitor of CREBBP/EP300 bromodomain,potent CAS# 1613695-14-9 |
- GSK 525768A
Catalog No.:BCC1603
CAS No.:1260530-25-3
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- MS436
Catalog No.:BCC4037
CAS No.:1395084-25-9
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
- Bromosporine
Catalog No.:BCC2226
CAS No.:1619994-69-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1613695-14-9 | SDF | Download SDF |
| PubChem ID | 72201027 | Appearance | Powder |
| Formula | C28H33ClN4O3 | M.Wt | 509.04 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 31 mg/mL (60.90 mM) *"≥" means soluble, but saturation unknown. | ||
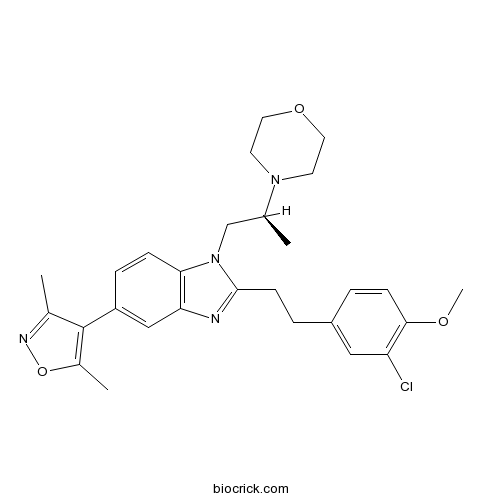
| Chemical Name | 4-[(2S)-1-[2-[2-(3-chloro-4-methoxyphenyl)ethyl]-5-(3,5-dimethyl-1,2-oxazol-4-yl)benzimidazol-1-yl]propan-2-yl]morpholine | ||
| SMILES | CC1=C(C(=NO1)C)C2=CC3=C(C=C2)N(C(=N3)CCC4=CC(=C(C=C4)OC)Cl)CC(C)N5CCOCC5 | ||
| Standard InChIKey | GEPYBHCJBORHCE-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C28H33ClN4O3/c1-18(32-11-13-35-14-12-32)17-33-25-8-7-22(28-19(2)31-36-20(28)3)16-24(25)30-27(33)10-6-21-5-9-26(34-4)23(29)15-21/h5,7-9,15-16,18H,6,10-14,17H2,1-4H3/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SGC-CBP30 is a potent inhibitor of CREBBP/EP300 with IC50 values of 21 nM and 38 nM, respectively. | |||||
| Targets | CREBBP | EP300 | ||||
| IC50 | 21 nM | 38 nM | ||||
| Cell experiment [1]: | |
| Cell lines | HeLa cells and RKO cells |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 37oC |
| Applications | In HeLa cells, treatment of SAHA-treated cells with 0.1 μM SGC-CBP30 reduces FRAP recovery times back to unstimulated levels. In RKO cells, SGC-CBP30 effectively inhibits the Doxorubicin induced p53 activity in a dose-dependent manner. |
| References: 1. Hay DA, Fedorov O, Martin S et al. Discovery and optimization of small-molecule ligands for the CBP/p300 bromodomains. J Am Chem Soc. 2014 Jul 2;136(26):9308-19. | |

SGC-CBP30 Dilution Calculator

SGC-CBP30 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9645 mL | 9.8224 mL | 19.6448 mL | 39.2896 mL | 49.1121 mL |
| 5 mM | 0.3929 mL | 1.9645 mL | 3.929 mL | 7.8579 mL | 9.8224 mL |
| 10 mM | 0.1964 mL | 0.9822 mL | 1.9645 mL | 3.929 mL | 4.9112 mL |
| 50 mM | 0.0393 mL | 0.1964 mL | 0.3929 mL | 0.7858 mL | 0.9822 mL |
| 100 mM | 0.0196 mL | 0.0982 mL | 0.1964 mL | 0.3929 mL | 0.4911 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SGC-CBP 30 is a selective inhibitor of CREBBP and EP300 with IC50 value of 21 nM and 38 nM, respectively [1].
EP300 (E1A binding protein p300) is encoded by EP300 gene and plays an important role in regulating cell growth and division, prompting cells mature and assume specialized functions, and preventing tumor cells growth. CREBBP (CREB-binding protein) is encoded by CREBBP gene and involves in the transcriptional coactivation of many different transcription factors [2].
References:
[1]. Duncan A. Hay, Oleg Fedorov, Sarah Martin, et al. Discovery and Optimization of Small-Molecule Ligands for the CBP/p300 Bromodomains [J]. J. Am. Chem. Soc., 2014, 136 (26), pp 9308–9319.
[2]. van Belzen M, Bartsch O, Lacombe D, et al. Rubinstein-Taybi syndrome (CREBBP, EP300) [J]. Eur J Hum Genet. 2011 Jan; 19(1):preceeding 118-20.
- Fmoc-Chg-OH
Catalog No.:BCC3164
CAS No.:161321-36-4
- 1-O-Acetyl-6-O-isobutyrylbritannilactone
Catalog No.:BCN7795
CAS No.:1613152-34-3
- SR-9243
Catalog No.:BCC3983
CAS No.:1613028-81-1
- H-Orn(Z)-OtBu.HCl
Catalog No.:BCC2677
CAS No.:161234-80-6
- Cimicidanol 3-O-alpha-L-arabinoside
Catalog No.:BCN6528
CAS No.:161207-05-2
- H-Asp(OEt)-OEt.HCl
Catalog No.:BCC2888
CAS No.:16115-68-7
- Bidwillol A
Catalog No.:BCN4858
CAS No.:161099-42-9
- Cimicifugoside H2
Catalog No.:BCN7949
CAS No.:161097-77-4
- Formyl-DL-Trp-OH
Catalog No.:BCC3120
CAS No.:16108-03-5
- Epimedokoreanin C
Catalog No.:BCN8080
CAS No.:161068-54-8
- Epimedokoreanin B
Catalog No.:BCN6483
CAS No.:161068-53-7
- VT-464
Catalog No.:BCC5398
CAS No.:1610537-15-9
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- ABT
Catalog No.:BCC7998
CAS No.:1614-12-6
- SIN-1 chloride
Catalog No.:BCC5670
CAS No.:16142-27-1
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
Discovery and optimization of 1-(1H-indol-1-yl)ethanone derivatives as CBP/EP300 bromodomain inhibitors for the treatment of castration-resistant prostate cancer.[Pubmed:29448139]
Eur J Med Chem. 2018 Mar 10;147:238-252.
The CREB (cAMP responsive element binding protein) binding protein (CBP) and its homolog EP300 have emerged as new therapeutic targets for the treatment of cancer and inflammatory diseases. Here we report the identification, optimization and evaluation of 1-(1H-indol-1-yl)ethanone derivatives as CBP/EP300 inhibitors starting from fragment-based virtual screening (FBVS). A cocrystal structure of the inhibitor (22e) in complex with CBP provides a solid structural basis for further optimization. The most potent compound 32h binds to the CBP bromodomain and has an IC50 value of 0.037muM in the AlphaScreen assay which was 2 times more potent than the reported CBP bromodomain inhibitor SGC-CBP30 in our hands. 32h also exhibit high selectivity for CBP/EP300 over other bromodomain-containing proteins. Notably, the ester derivative (29h) of compound 32h markedly inhibits cell growth in several prostate cancer cell lines including LNCaP, 22Rv1 and LNCaP derived C4-2B. Compound 29h suppresses the mRNA expression of full length AR (AR-FL), AR target genes and other oncogene in LNCaP cells. 29h also reduces the expression of PSA, the biomarker of prostate cancer. CBP/EP300 inhibitor 29h represents a promising lead compound for the development of new therapeutics for the treatment of castration-resistant prostate cancer.
Activity of bromodomain protein inhibitors/binders against asexual-stage Plasmodium falciparum parasites.[Pubmed:29631126]
Int J Parasitol Drugs Drug Resist. 2018 Aug;8(2):189-193.
Bromodomain-containing proteins (BDPs) are involved in the regulation of eukaryotic gene expression. Compounds that bind and/or inhibit BDPs are of interest as tools to better understand epigenetic regulation, and as possible drug leads for different diseases, including malaria. In this study, we assessed the activity of 42 compounds demonstrated or predicted (using virtual screening of a pharmacophore model) to bind/inhibit eukaryotic BDPs for activity against Plasmodium falciparum malaria parasites. In silico docking studies indicated that all compounds are predicted to participate in a typical hydrogen bond interaction with the conserved asparagine (Asn1436) of the P. falciparum histone acetyltransferase (PfGCN5) bromodomain and a conserved water molecule. Only one compound (the dimethylisoxazole SGC-CBP30; a selective inhibitor of CREBBP (CBP) and EP300 bromodomains) is also predicted to have a salt-bridge between the morpholine nitrogen and Glu1389. When tested for in vitro activity against asynchronous asexual stage P. falciparum Dd2 parasites, all compounds displayed 50% growth inhibitory concentrations (IC50) >10muM. Further testing of the three most potent compounds using synchronous parasites for 72h showed that SGC-CBP30 was the most active (IC50 3.2muM). In vitro cytotoxicity assays showed that SGC-CBP30 has approximately 7-fold better selectivity for the parasites versus a human cell line (HEK 293). Together these data provide a possible starting point for future investigation of these, or related compounds, as tools to understand epigenetic regulation or as potential new drug leads.


