2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidineCAS# 161599-46-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 161599-46-8 | SDF | Download SDF |
| PubChem ID | 11809635 | Appearance | Powder |
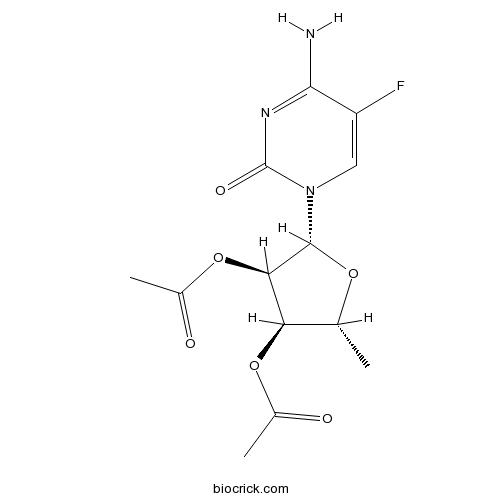
| Formula | C13H16FN3O6 | M.Wt | 329.28 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3R,4R,5R)-4-acetyloxy-5-(4-amino-5-fluoro-2-oxopyrimidin-1-yl)-2-methyloxolan-3-yl] acetate | ||
| SMILES | CC1C(C(C(O1)N2C=C(C(=NC2=O)N)F)OC(=O)C)OC(=O)C | ||
| Standard InChIKey | NWJBWNIUGNXJGO-RPULLILYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine Dilution Calculator

2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0369 mL | 15.1846 mL | 30.3693 mL | 60.7386 mL | 75.9232 mL |
| 5 mM | 0.6074 mL | 3.0369 mL | 6.0739 mL | 12.1477 mL | 15.1846 mL |
| 10 mM | 0.3037 mL | 1.5185 mL | 3.0369 mL | 6.0739 mL | 7.5923 mL |
| 50 mM | 0.0607 mL | 0.3037 mL | 0.6074 mL | 1.2148 mL | 1.5185 mL |
| 100 mM | 0.0304 mL | 0.1518 mL | 0.3037 mL | 0.6074 mL | 0.7592 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- SIN-1 chloride
Catalog No.:BCC5670
CAS No.:16142-27-1
- ABT
Catalog No.:BCC7998
CAS No.:1614-12-6
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- Fmoc-Chg-OH
Catalog No.:BCC3164
CAS No.:161321-36-4
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
- Hispidanin B
Catalog No.:BCN7394
CAS No.:1616080-84-2
- VRT-1353385
Catalog No.:BCC6433
CAS No.:1616113-45-1
- EPZ015666
Catalog No.:BCC5653
CAS No.:1616391-65-1
- Erythrinin D
Catalog No.:BCN6858
CAS No.:1616592-59-6
- 1,5-difluoro-3-methyl-2-nitrobenzene
Catalog No.:BCN6404
CAS No.:1616526-80-7
- Erythrinin G
Catalog No.:BCN6857
CAS No.:1616592-61-0
- Erythrinin H
Catalog No.:BCN6870
CAS No.:1616592-62-1
- ONC201
Catalog No.:BCC3989
CAS No.:1616632-77-9
- Dodovisone A
Catalog No.:BCN6839
CAS No.:1616683-50-1
Cytotoxic gold(iii) complexes incorporating a 2,2':6',2''-terpyridine ligand framework - the impact of the substituent in the 4'-position of a terpy ring.[Pubmed:28234396]
Dalton Trans. 2017 Mar 7;46(10):3381-3392.
Two gold(iii) complexes incorporating 2,2':6',2''-terpyridine derivatives have been synthesised and characterized, and the possibility of tuning the cytotoxic activity by structural modifications of a terpy ligand has been examined. Both complexes [AuCl(4'-R(1)-terpy)](PF6)2 (1) and [AuCl(4'-R(2)-terpy)](PF6)2 (2), where R(1) is 2-pyridyl and R(2) is 3-pyridyl, show good anti-proliferative activities against HCT116, which are higher in relation to those of the free ligands, [AuCl(terpy)](PF6)2 and standard anticancer drug cisplatin. The cytotoxic properties of the gold complexes were examined by MTS assay, cell cycle and apoptosis analysis, ROS measurements, determination of mitochondrial membrane potential and mass, and staining of phosphatidylserine with Annexin-V antibody FITC-conjugated together with PI.
Characterization and cytotoxic effect of aqua-(2,2',2''-nitrilotriacetato)-oxo-vanadium salts on human osteosarcoma cells.[Pubmed:28204978]
Biometals. 2017 Apr;30(2):261-275.
The use of protonated N-heterocyclic compound, i.e. 2,2'-bipyridinium cation, [bpyH(+)], enabled to obtain the new nitrilotriacetate oxidovanadium(IV) salt of the stoichiometry [bpyH][VO(nta)(H2O)]H2O. The X-ray measurements have revealed that the compound comprises the discrete mononuclear [VO(nta)(H2O)](-) coordination ion that can be rarely found among other known compounds containing nitrilotriacetate oxidovanadium(IV) moieties. The antitumor activity of [bpyH][VO(nta)(H2O)]H2O and its phenanthroline analogue, [phenH][VO(nta)(H2O)](H2O)0.5, towards human osteosarcoma cell lines (MG-63 and HOS) has been assessed (the LDH and BrdU tests) and referred to cis-Pt(NH3)2Cl2 (used as a positive control). The compounds exert a stronger cytotoxic effect on MG-63 and HOS cells than in untransformed human osteoblast cell line. Thus, the [VO(nta)(H2O)](-) containing coordination compounds can be considered as possible antitumor agents in the osteosarcoma model of bone-related cells in culture.
High Prevalence and Predominance of the aph(2'')-If Gene Conferring Aminoglycoside Resistance in Campylobacter.[Pubmed:28264854]
Antimicrob Agents Chemother. 2017 Apr 24;61(5). pii: AAC.00112-17.
Campylobacter is a major foodborne pathogen, and previous studies revealed that Campylobacter isolates from food-producing animals are increasingly resistant to gentamicin in China. The molecular epidemiology and genetic mechanisms responsible for gentamicin resistance in China have not been well understood. In this study, 607 Campylobacter isolates of chicken and swine origins collected in 2014 were analyzed, revealing that 15.6% (25/160) of the Campylobacter jejuni isolates and 79.9% (357/447) of the Campylobacter coli isolates were resistant to gentamicin. PCR detection of the gentamicin resistance genes indicated that aph(2'')-If was more prevalent than the previously identified aacA/aphD gene and has become the dominant gentamicin resistance determinant in Campylobacter Transformation and whole-genome sequencing as well as long-range PCR discovered that aph(2'')-If was located on a chromosomal segment inserted between two conserved genes, Cj0299 and panB Cloning of aph(2'')-If into gentamicin-susceptible C. jejuni NCTC 11168 confirmed its function in conferring high-level resistance to gentamicin and kanamycin. Molecular typing by pulsed-field gel electrophoresis suggested that both regional expansion of a particular clone and horizontal transmission were involved in the dissemination of the aph(2'')-If gene in Campylobacter To our knowledge, this is the first report describing the high prevalence of a chromosomally encoded aph(2'')-If gene in Campylobacter The high prevalence and predominance of this gene might be driven by the use of aminoglycoside antibiotics in food animal production in China and potentially compromise the usefulness of gentamicin as a therapeutic agent for Campylobacter-associated systemic infection.
Discovery of 4-((3'R,4'S,5'R)-6''-Chloro-4'-(3-chloro-2-fluorophenyl)-1'-ethyl-2''-oxodispiro[ cyclohexane-1,2'-pyrrolidine-3',3''-indoline]-5'-carboxamido)bicyclo[2.2.2]octane -1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development.[Pubmed:28339198]
J Med Chem. 2017 Apr 13;60(7):2819-2839.
We previously reported the design of spirooxindoles with two identical substituents at the carbon-2 of the pyrrolidine core as potent MDM2 inhibitors. In this paper we describe an extensive structure-activity relationship study of this class of MDM2 inhibitors, which led to the discovery of 60 (AA-115/APG-115). Compound 60 has a very high affinity to MDM2 (Ki < 1 nM), potent cellular activity, and an excellent oral pharmacokinetic profile. Compound 60 is capable of achieving complete and long-lasting tumor regression in vivo and is currently in phase I clinical trials for cancer treatment.


