Ro 48-8071CAS# 161582-11-2 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- WHI-P180 hydrochloride
Catalog No.:BCC4243
CAS No.:153437-55-9
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- RGB-286638
Catalog No.:BCC5519
CAS No.:784210-87-3
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 161582-11-2 | SDF | Download SDF |
| PubChem ID | 1949 | Appearance | Powder |
| Formula | C23H27BrFNO2 | M.Wt | 448.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >14.6mg/mL in DMSO | ||
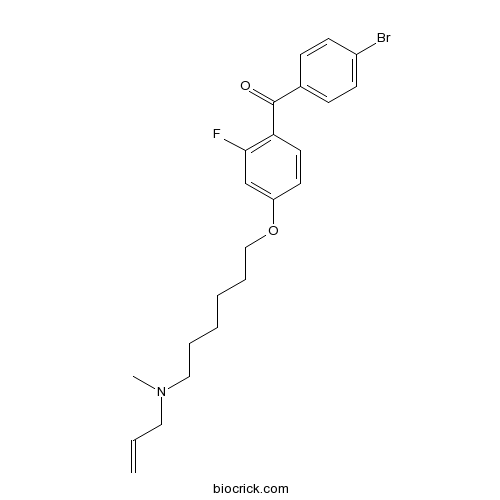
| Chemical Name | (4-bromophenyl)-[2-fluoro-4-[6-[methyl(prop-2-enyl)amino]hexoxy]phenyl]methanone | ||
| SMILES | CN(CCCCCCOC1=CC(=C(C=C1)C(=O)C2=CC=C(C=C2)Br)F)CC=C | ||
| Standard InChIKey | CMYCCJYVZIMDFU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ro 48-8071 Dilution Calculator

Ro 48-8071 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2303 mL | 11.1515 mL | 22.303 mL | 44.606 mL | 55.7575 mL |
| 5 mM | 0.4461 mL | 2.2303 mL | 4.4606 mL | 8.9212 mL | 11.1515 mL |
| 10 mM | 0.223 mL | 1.1152 mL | 2.2303 mL | 4.4606 mL | 5.5758 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.4461 mL | 0.8921 mL | 1.1152 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.4461 mL | 0.5576 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ro 48-8071 is an inhibitor of OSC (Oxidosqualene cyclase) with IC50 of appr 6.5 nM.
In Vitro:In HepG2 cells, Ro 48-8071 reduces cholesterol synthesis dose dependently with an IC50 value of appr 1.5 nM[1]. Ro 48-8071 (10 μM) significantly reduces the viability of PC-3 prostate cancer cells, but not normal prostate cells. Ro 48-8071 (10-30 μM) induces apoptosis of both LNCaP and C4-2 cell lines in a dose-dependent manner. And castration-resistant PC-3 and DU145 cells also demonstrate significant levels of apoptosis following 24-hour treatment with Ro 48-8071. Ro 48-8071 (10-25 μM) reduces AR protein expression in a dose-dependent manner. Ro 48-8071 (0.1-1 μM) increases ERβ protein expression dose-dependently in both hormone-dependent LNCaP and castration-resistant PC-3 cells[2]. Using mammalian cells engineered to express human ERα or ERβ protein, together with an ER-responsive luciferase promoter, Ro 48-8071 dose-dependently inhibits 17β-estradiol (E2)-induced ERα responsive luciferase activity (IC50, appr 10 µM), under conditions that are non-toxic to the cells[3].
In Vivo:Ro 48-8071 lowers LDL-C maximally appr 60% at 150 μmol/kg per day, with no further reduction up to 300 μmol/kg per day, leaving HDL-C unchanged at all doses in hamsters. Ro 48-8071 (≥00 μmol/kg per day) increases the amount of MOS in liver of hamsters. Ro 48-8071 (300 μmol/kg per day) remarkedly and significantly reduces VLDL secretion of hamsters[1]. Ro 48-8071 (5 or 20 mg/kg) significantly reduces in vivo tumor growth in mice, without weight loss of the mice. Furthermore, Ro 48-8071 at a concentration of 20 mg/kg, completely eradicates two of the 12 tumors being monitored in the mice in the timeframe tested[2]. Ro 48-8071 (20 mg/day/kg body weight) leads to a rapid and sustained inhibition (>50%) of cholesterol synthesis in the whole small intestine of BALB/c mice. Sterol synthesis is also reduced in the large intestine and stomach[4].
References:
[1]. Morand OH, et al. Ro 48-8.071, a new 2,3-oxidosqualene:lanosterol cyclase inhibitor lowering plasma cholesterol in hamsters, squirrel monkeys, and minipigs: comparison to simvastatin. J Lipid Res. 1997 Feb;38(2):373-90.
[2]. Liang Y, et al. Cholesterol biosynthesis inhibitor RO 48-8071 suppresses growth of hormone-dependent and castration-resistant prostate cancer cells. Onco Targets Ther. 2016 May 30;9:3223-32.
[3]. Liang Y, et al. Cholesterol biosynthesis inhibitors as potent novel anti-cancer agents: suppression of hormone-dependent breast cancer by the oxidosqualene cyclase inhibitor RO 48-8071. Breast Cancer Res Treat. 2014 Jul;146(1):51-62.
[4]. Chuang JC, et al. Sustained and selective suppression of intestinal cholesterol synthesis by Ro 48-8071, an inhibitor of 2,3-oxidosqualene:lanosterol cyclase, in the BALB/c mouse. Biochem Pharmacol. 2014 Apr 1;88(3):351-63.
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- SIN-1 chloride
Catalog No.:BCC5670
CAS No.:16142-27-1
- ABT
Catalog No.:BCC7998
CAS No.:1614-12-6
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- Fmoc-Chg-OH
Catalog No.:BCC3164
CAS No.:161321-36-4
- 1-O-Acetyl-6-O-isobutyrylbritannilactone
Catalog No.:BCN7795
CAS No.:1613152-34-3
- SR-9243
Catalog No.:BCC3983
CAS No.:1613028-81-1
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
- Hispidanin B
Catalog No.:BCN7394
CAS No.:1616080-84-2
- VRT-1353385
Catalog No.:BCC6433
CAS No.:1616113-45-1
- EPZ015666
Catalog No.:BCC5653
CAS No.:1616391-65-1
- Erythrinin D
Catalog No.:BCN6858
CAS No.:1616592-59-6
- 1,5-difluoro-3-methyl-2-nitrobenzene
Catalog No.:BCN6404
CAS No.:1616526-80-7
- Erythrinin G
Catalog No.:BCN6857
CAS No.:1616592-61-0
- Erythrinin H
Catalog No.:BCN6870
CAS No.:1616592-62-1
Cholesterol biosynthesis inhibitors as potent novel anti-cancer agents: suppression of hormone-dependent breast cancer by the oxidosqualene cyclase inhibitor RO 48-8071.[Pubmed:24878988]
Breast Cancer Res Treat. 2014 Jul;146(1):51-62.
In most human breast cancers, tumor cell proliferation is estrogen dependent. Although hormone-responsive tumors initially respond to anti-estrogen therapies, most of them eventually develop resistance. Our goal was to identify alternative targets that might be regulated to control breast cancer progression. Sulforhodamine B assay was used to measure the viability of cultured human breast cancer cell lines exposed to various inhibitors. Protein expression in whole-cell extracts was determined by Western blotting. BT-474 tumor xenografts in nude mice were used for in vivo studies of tumor progression. Ro 48-8071 ([4'-[6-(Allylmethylamino)hexyloxy]-4-bromo-2'-fluorobenzophenone fumarate]; RO), a small-molecule inhibitor of oxidosqualene cyclase (OSC, a key enzyme in cholesterol biosynthesis), potently reduced breast cancer cell viability. In vitro exposure of estrogen receptor (ER)-positive human breast cancer cells to pharmacological levels of RO or a dose close to the IC50 for OSC (nM) reduced cell viability. Administration of RO to mice with BT-474 tumor xenografts prevented tumor growth, with no apparent toxicity. RO degraded ERalpha while concomitantly inducing the anti-proliferative protein ERbeta. Two other cholesterol-lowering drugs, Fluvastatin and Simvastatin, were less effective in reducing breast cancer cell viability and were found not to induce ERbeta. ERbeta inhibition or knockdown prevented RO-dependent loss of cell viability. Importantly, RO had no effect on the viability of normal human mammary cells. RO is a potent inhibitor of hormone-dependent human breast cancer cell proliferation. The anti-tumor properties of RO appear to be in part due to an off-target effect that increases the ratio of ERbeta/ERalpha in breast cancer cells.
Sustained and selective suppression of intestinal cholesterol synthesis by Ro 48-8071, an inhibitor of 2,3-oxidosqualene:lanosterol cyclase, in the BALB/c mouse.[Pubmed:24486573]
Biochem Pharmacol. 2014 Apr 1;88(3):351-63.
The small intestine plays a fundamentally important role in regulating whole body cholesterol balance and plasma lipoprotein composition. This is articulated through the interplay of a constellation of genes that ultimately determines the net amount of chylomicron cholesterol delivered to the liver. Major advances in our insights into regulation of the cholesterol absorption pathway have been made using genetically manipulated mouse models and agents such as ezetimibe. One unresolved question is how a sustained pharmacological inhibition of intestinal cholesterol synthesis in vivo may affect cholesterol handling by the absorptive cells. Here we show that the lanosterol cyclase inhibitor, Ro 48-8071, when fed to BALB/c mice in a chow diet (20 mg/day/kg body weight), leads to a rapid and sustained inhibition (>50%) of cholesterol synthesis in the whole small intestine. Sterol synthesis was also reduced in the large intestine and stomach. In contrast, hepatic cholesterol synthesis, while markedly suppressed initially, rebounded to higher than baseline rates within 7 days. Whole body cholesterol synthesis, fractional cholesterol absorption, and fecal neutral and acidic sterol excretion were not consistently changed with Ro 48-8071 treatment. There were no discernible effects of this agent on intestinal histology as determined by H&E staining and the level of Ki67, an index of proliferation. The mRNA expression for multiple genes involved in intestinal cholesterol regulation including NPC1L1 was mostly unchanged although there was a marked rise in the mRNA level for the PXR target genes CYP3A11 and CES2A.
Cholesterol synthesis inhibitor RO 48-8071 suppresses transcriptional activity of human estrogen and androgen receptor.[Pubmed:25051231]
Oncol Rep. 2014 Oct;32(4):1727-33.
Breast cancer cells express enzymes that convert cholesterol, the synthetic precursor of steroid hormones, into estrogens and androgens, which then drive breast cancer cell proliferation. In the present study, we sought to determine whether oxidosqualene cyclase (OSC), an enzyme in the cholesterol biosynthetic pathway, may be targeted to suppress progression of breast cancer cells. In previous studies, we showed that the OSC inhibitor Ro 48-8071 (RO) may be a ligand which could potentially be used to control the progression of estrogen receptor-alpha (ERalpha)-positive breast cancer cells. Herein, we showed, by real-time PCR analysis of mRNA from human breast cancer biopsies, no significant differences in OSC expression at various stages of disease, or between tumor and normal mammary cells. Since the growth of hormone-responsive tumors is ERalpha-dependent, we conducted experiments to determine whether RO affects ERalpha. Using mammalian cells engineered to express human ERalpha or ERbeta protein, together with an ER-responsive luciferase promoter, we found that RO dose-dependently inhibited 17beta-estradiol (E2)-induced ERalpha responsive luciferase activity (IC50 value, ~10 microM), under conditions that were non-toxic to the cells. RO was less effective against ERbeta-induced luciferase activity. Androgen receptor (AR) mediated transcriptional activity was also reduced by RO. Notably, while ERalpha activity was reduced by atorvastatin, the HMG-CoA reductase inhibitor did not influence AR activity, showing that RO possesses broader antitumor properties. Treatment of human BT-474 breast cancer cells with RO reduced levels of estrogen-induced PR protein, confirming that RO blocks ERalpha activity in tumor cells. Our findings demonstrate that an important means by which RO suppresses hormone-dependent growth of breast cancer cells is through its ability to arrest the biological activity of ERalpha. This warrants further investigation of RO as a potential therapeutic agent for use against hormone-dependent breast cancers.
Cholesterol biosynthesis inhibitor RO 48-8071 suppresses growth of hormone-dependent and castration-resistant prostate cancer cells.[Pubmed:27313468]
Onco Targets Ther. 2016 May 30;9:3223-32.
Standard treatment for primary prostate cancer includes systemic exposure to chemotherapeutic drugs that target androgen receptor or antihormone therapy (chemical castration); however, drug-resistant cancer cells generally emerge during treatment, limiting the continued use of systemic chemotherapy. Patients are then treated with more toxic standard therapies. Therefore, there is an urgent need for novel and more effective treatments for prostate cancer. The cholesterol biosynthetic pathway is an attractive therapeutic target for treating endocrine-dependent cancers because cholesterol is an essential structural and functional component of cell membranes as well as the metabolic precursor of endogenous steroid hormones. In this study, we have examined the effects of Ro 48-8071 (4'-[6-(allylmethylamino)hexyloxy]-4-bromo-2'-fluorobenzophenone fumarate; Roche Pharmaceuticals internal reference: RO0488071) (RO), which is an inhibitor of 2, 3-oxidosqualene cyclase (a key enzyme in the cholesterol biosynthetic pathway), on prostate cancer cells. Exposure of both hormone-dependent and castration-resistant human prostate cancer cells to RO reduced prostate cancer cell viability and induced apoptosis in vitro. RO treatment reduced androgen receptor protein expression in hormone-dependent prostate cancer cells and increased estrogen receptor beta (ERbeta) protein expression in both hormone-dependent and castration-resistant prostate cancer cell lines. Combining RO with an ERbeta agonist increased its ability to reduce castration-resistant prostate cancer cell viability. In addition, RO effectively suppressed the growth of aggressive castration-resistant human prostate cancer cell xenografts in vivo without any signs of toxicity to experimental animals. Importantly, RO did not reduce the viability of normal prostate cells in vitro. Our study is the first to demonstrate that the cholesterol biosynthesis inhibitor RO effectively suppresses growth of human prostate cancer cells. Our findings suggest that cholesterol biosynthesis inhibitors such as RO, when used in combination with commonly used chemotherapeutic drugs or ERbeta specific ligands, could represent a novel therapeutic approach to prevent the growth of prostate cancer tumors.


