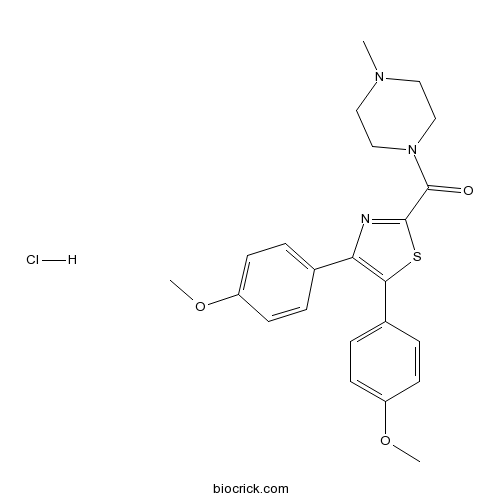
FR 122047 hydrochlorideCyclooxygenase (COX-1) inhibitor CAS# 130717-51-0 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Fumonisin B1
Catalog No.:BCC2461
CAS No.:116355-83-0
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130717-51-0 | SDF | Download SDF |
| PubChem ID | 196840 | Appearance | Powder |
| Formula | C23H26ClN3O3S | M.Wt | 459.99 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water | ||
| Chemical Name | [4,5-bis(4-methoxyphenyl)-1,3-thiazol-2-yl]-(4-methylpiperazin-1-yl)methanone;hydrochloride | ||
| SMILES | CN1CCN(CC1)C(=O)C2=NC(=C(S2)C3=CC=C(C=C3)OC)C4=CC=C(C=C4)OC.Cl | ||
| Standard InChIKey | YWMAVHIKOAOSFM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H25N3O3S.ClH/c1-25-12-14-26(15-13-25)23(27)22-24-20(16-4-8-18(28-2)9-5-16)21(30-22)17-6-10-19(29-3)11-7-17;/h4-11H,12-15H2,1-3H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective cyclooxygenase-1 (COX-1) inhibitor (IC50 values are 0.028 and 65 μM for COX-1 and COX-2 respectively). Antiplatelet, analgesic and anti-inflammatory following oral administration in vivo. |

FR 122047 hydrochloride Dilution Calculator

FR 122047 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.174 mL | 10.8698 mL | 21.7396 mL | 43.4792 mL | 54.349 mL |
| 5 mM | 0.4348 mL | 2.174 mL | 4.3479 mL | 8.6958 mL | 10.8698 mL |
| 10 mM | 0.2174 mL | 1.087 mL | 2.174 mL | 4.3479 mL | 5.4349 mL |
| 50 mM | 0.0435 mL | 0.2174 mL | 0.4348 mL | 0.8696 mL | 1.087 mL |
| 100 mM | 0.0217 mL | 0.1087 mL | 0.2174 mL | 0.4348 mL | 0.5435 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SDZ WAG 994
Catalog No.:BCC7374
CAS No.:130714-47-5
- (R)-(+)-Propranolol hydrochloride
Catalog No.:BCC6810
CAS No.:13071-11-9
- Dorzolamide HCl
Catalog No.:BCC2311
CAS No.:130693-82-2
- RP 001 hydrochloride
Catalog No.:BCC7905
CAS No.:1306761-53-4
- Ozanimod (RPC1063)
Catalog No.:BCC6533
CAS No.:1306760-87-1
- PD123319
Catalog No.:BCC5010
CAS No.:130663-39-7
- Bindarit
Catalog No.:BCC4965
CAS No.:130641-38-2
- N-(2-Hydroxyethyl)-3-(4-nitrophenyl)propylamine
Catalog No.:BCC9053
CAS No.:130634-09-2
- (R)-(+)-Corypalmine
Catalog No.:BCN2289
CAS No.:13063-54-2
- Nitidine chloride
Catalog No.:BCN4957
CAS No.:13063-04-2
- Yangambin
Catalog No.:BCN6706
CAS No.:13060-14-5
- alpha-Terthienylmethanol
Catalog No.:BCN6161
CAS No.:13059-93-3
- Decloxizine dihydrochloride
Catalog No.:BCC5549
CAS No.:13073-96-6
- m-CPP hydrochloride
Catalog No.:BCC5680
CAS No.:13078-15-4
- 6-O-benzoylgomisin O
Catalog No.:BCN3092
CAS No.:130783-32-3
- MDL-29951
Catalog No.:BCC4059
CAS No.:130798-51-5
- Sipatrigine
Catalog No.:BCC7847
CAS No.:130800-90-7
- Pseudolarifuroic acid
Catalog No.:BCN8048
CAS No.:130825-79-5
- 7-Oxohinokinin
Catalog No.:BCN6162
CAS No.:130837-92-2
- Scoparinol
Catalog No.:BCN6163
CAS No.:130838-00-5
- Decursinol angelate
Catalog No.:BCC9222
CAS No.:130848-06-5
- ent-kaurane-3,16,17-triol
Catalog No.:BCN6164
CAS No.:130855-22-0
- 1beta,10beta-Epoxydehydroleucodin
Catalog No.:BCN7331
CAS No.:130858-00-3
- Fmoc-Val-OSu
Catalog No.:BCC3572
CAS No.:130878-68-1
Differential effect of FR122047, a selective cyclo-oxygenase-1 inhibitor, in rat chronic models of arthritis.[Pubmed:11834626]
Br J Pharmacol. 2002 Feb;135(3):782-8.
We investigated the effects of FR122047 (1-[(4,5-bis(4-methoxyphenyl)-2-thiazoyl)carbonyl]-4-methylpiperazine hydrochloride), a selective cyclo-oxygenase (COX)-1 inhibitor, in rat type II collagen-induced arthritis (CIA) and adjuvant-induced arthritis (AIA). Using an ex vivo rat whole blood assay, FR122047 (0.032 - 3.2 mg kg(-1)) inhibited COX-1-derived thromboxane (TX) B(2) production with ED(50) value of 0.059 mg kg(-1), indicating that it was orally active, but did not inhibit lipopolysaccharide-induced prostaglandin (PG) E(2) production derived by COX-2. Oral administration of FR122047 showed a dose-dependent anti-inflammatory effect in rat CIA with ED(50) value of 0.56 mg kg(-1). This drug also dose dependently suppressed the levels of PGE(2) and TXB(2) in CIA rat paws with ED(50) values of 0.24 and 0.13 mg kg(-1), respectively. FR122047 had no effect in rat AIA model. In contrast, indomethacin, a non-selective COX inhibitor, was anti-inflammatory and reduced the formation of PGs in AIA rat paws. Unlike indomethacin, chronic treatment of FR122047 did not damage the stomach mucosa in CIA rats. These results demonstrate that COX-1 contributes to the oedema and the formation of PGE(2) and TXB(2) in rat CIA model, but not in rat AIA model. We conclude that FR122047 has an orally active and anti-inflammatory effect mediated by inhibition of PGE(2) and TXB(2) produced by COX-1 at a site of inflammation induced by type II collagen and it may be a useful tool for studying the involvement of COX-1 in various in vivo models of inflammation.
The analgesic effect profile of FR122047, a selective cyclooxygenase-1 inhibitor, in chemical nociceptive models.[Pubmed:10720634]
Eur J Pharmacol. 2000 Mar 10;391(1-2):49-54.
The pharmacological profile of the analgesic agent, 1-[(4, 5-bis(4-methoxyphenyl)-2-thiazoyl)carbonyl]-4-methylpiperazine hydrochloride (FR122047), was investigated. In recombinant human cyclooxygenase enzyme assays, the inhibition of prostaglandin E(2) formation by FR122047 was 2300 times more selective for cyclooxygenase-1 than cyclooxygenase-2. Oral administration of FR122047 (3.2-100 mg/kg) dose dependently reduced the phase 2 response (10-60 min) of the formalin test in rats. This effect was 3 times less potent than that of indomethacin. FR122047 (1-32 mg/kg; p. o.) showed a dose-dependent analgesic effect against the acetic acid-induced writhing response in mice. Furthermore, FR122047 (0. 01-10 mg/kg, p.o.) inhibited the increase in 6-keto prostaglandin F(1alpha) level in acetic acid-injected mouse peritoneal cavity. However, a selective cyclooxygenase-2 inhibitor, NS-398, had no effect in these cyclooxygenase-1 sensitive pain models. These results suggest that FR122047, a selective cyclooxygenase-1 inhibitor, shows an analgesic effect in chemical nociceptive models and may be a useful analgesic agent.
Antiplatelet agents based on cyclooxygenase inhibition without ulcerogenesis. Evaluation and synthesis of 4,5-bis(4-methoxyphenyl)-2-substituted-thiazoles.[Pubmed:8164261]
J Med Chem. 1994 Apr 15;37(8):1189-99.
The syntheses, biological evaluations, and structure-activity relationships of a series of 4,5-bis-(4-methoxyphenyl)-2-substituted-thiazoles as potent antiplatelet agents with vasodilatory activity are described. 2-Guanidino-4,5-bis(4-methoxyphenyl)thiazole (3), designed from two parent compounds (itazigrel and timegadine), showed inhibitory activity of malondialdehyde (MDA, IC50 = 31 microM) production which is formed from the cyclooxygenase (CO)-catalyzed oxygenation of arachidonic acid in the synthesis of prostanoids in platelets, with vasodilatory activity (ED50 = 2.0 microM). Further structure-activity relationship studies on 3 culminated in the preparation of 4,5-bis(4-methoxyphenyl)-2-[(1-methylpiperazin-4-yl)carbonyl]thiaz ole (10a, FR122047) which exhibited potent inhibitory activity on MDA synthesis in vitro (IC50 = 0.088 microM) and platelet aggregation in guinea pigs ex vivo (100% inhibition even 6 h after 1.0 mg/kg administration) with vasodilatory activity in vitro (ED50 = 6.2 microM). Moreover, 10a demonstrated no ulcerogenesis effect in rats even at 100 mg/kg dosage (safety margin in rats is more than 70 while that of aspirin is only 1.2) in spite of its potent CO inhibition (IC50 = 0.43 microM14), while the use of aspirin, a CO inhibitor and the most popular thromboembolic drug, is restricted by the side effect. Pharmacokinetic studies on 10a have revealed that 10a is detectable in platelet-rich plasma but not in platelet-poor plasma 1 day after oral administration, which indicates that 10a tends to be localized in platelets. This property could be responsible for its low toxicity and reduction of side effects in clinical studies.
The anti-platelet actions of FR122047, a novel cyclooxygenase inhibitor.[Pubmed:8276067]
Eur J Pharmacol. 1993 Oct 19;243(2):179-84.
The anti-platelet actions of 1-[(4,5-bis(4-methoxyphenyl)-2- thiazoyl)carbonyl]-4-methylpiperazine hydrochloride (FR122047) were investigated in vitro and in vivo. FR122047 was 100 times more potent than aspirin against arachidonic acid- and collagen-induced human and guinea-pig platelet aggregation in vitro. Its actions on platelets were a result of cyclooxygenase inhibition. The single oral dose of FR122047 inhibited arachidonic acid- and collagen-induced aggregation with an ED50 of 280 micrograms/kg and 530 micrograms/kg, respectively, in guinea-pigs. The anti-platelet action was augmented 5-10 times by repeated administration for 4 days. At 1 mg/kg the inhibitory actions were prolonged for 48 h and the drug concentration was < 0.1 ng/ml in platelet-poor plasma at 24 h and 0.282 ng/ml in platelet-rich plasma at 48 h. The safety margin in rats (minimum ulcerogenic dose/ED50 for anti-platelet aggregation) of FR122047 was more than 70, while that of aspirin was only 1.2. These results indicate that FR122047 is concentrated in platelets and may be a useful anti-platelet agent.


