FosaprepitantNeurokinin-1 antagonist CAS# 172673-20-0 |
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 172673-20-0 | SDF | Download SDF |
| PubChem ID | 219090 | Appearance | Powder |
| Formula | C23H22F7N4O6P | M.Wt | 614.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L-758298 | ||
| Solubility | >30.5mg/mL in DMSO | ||
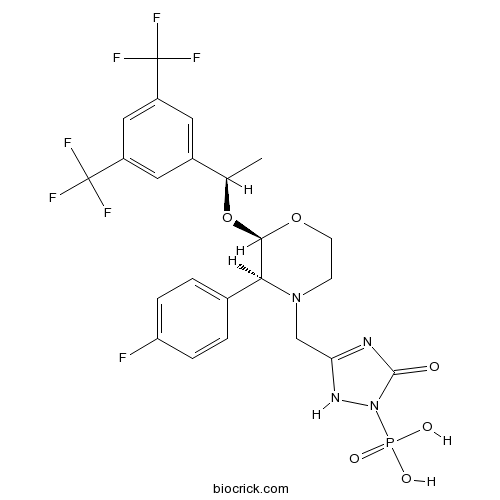
| Chemical Name | [5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-3-oxo-1H-1,2,4-triazol-2-yl]phosphonic acid | ||
| SMILES | CC(C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)OC2C(N(CCO2)CC3=NC(=O)N(N3)P(=O)(O)O)C4=CC=C(C=C4)F | ||
| Standard InChIKey | BARDROPHSZEBKC-OITMNORJSA-N | ||
| Standard InChI | InChI=1S/C23H22F7N4O6P/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)40-20-19(13-2-4-17(24)5-3-13)33(6-7-39-20)11-18-31-21(35)34(32-18)41(36,37)38/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H,31,32,35)(H2,36,37,38)/t12-,19+,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fosaprepitant (L-758298) is a neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting.
IC50 Value:
Target: NK1 receptor
in vitro: Fosaprepitant (also known as MK-0517 and L-758,298) is a water-soluble phosphoryl prodrug for aprepitant, which, when administered intravenously, is converted to aprepitant within 30 min of intravenous administration via the action of ubiquitous phosphatases. Owing to the rapid conversion offosaprepitant to the active form (aprepitant), fosaprepitant 115 mg provided the same aprepitant exposure in terms of AUC as aprepitant 12 mg orally, and fosaprepitant is expected to provide a correspondingly similar antiemetic effect as aprepitant [1].
in vivo: Fosaprepitant is well tolerated with mild to moderate venous irritation being the only additional toxicity to those seen with oral aprepitant, and that is a function of dose, concentration, and infusion rate [2]. Patients receiving cisplatin ≥ 70 mg/m(2) for the first time received ondansetron and dexamethasone with a standard aprepitant regimen (125 mg on day 1, 80 mg on day 2, 80 mg on day 3) or a single-dose fosaprepitant regimen (150 mg on day 1) [3]. Single-dose fosaprepitant used in combination with granisetron and dexamethasone was well-tolerated and effective in preventing chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic cancer chemotherapy, including high-dose cisplatin [4]. References: | |||||

Fosaprepitant Dilution Calculator

Fosaprepitant Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6276 mL | 8.1379 mL | 16.2758 mL | 32.5516 mL | 40.6894 mL |
| 5 mM | 0.3255 mL | 1.6276 mL | 3.2552 mL | 6.5103 mL | 8.1379 mL |
| 10 mM | 0.1628 mL | 0.8138 mL | 1.6276 mL | 3.2552 mL | 4.0689 mL |
| 50 mM | 0.0326 mL | 0.1628 mL | 0.3255 mL | 0.651 mL | 0.8138 mL |
| 100 mM | 0.0163 mL | 0.0814 mL | 0.1628 mL | 0.3255 mL | 0.4069 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fosaprepitant (L-758,298 MK-0517) is an antagonist of neurokinin-1[1].
Fosaprepitant has shown the pharmacodynamic function by its active metabolite aprepitant. Fosaprepitanot has been found to be a highly selective antagonist of the NK-1 receptor and inhibit the cation of substance P. In addition, Fosaprepitant has been exhibited to have the effect on cisplatin induced emesis in the classical ferret model. Besides, because of the brain penetrating of aprepitant, Fosaprepitant has been revealed to have a very high affinity for the NK-1 receptor and increase the efficacy by dexamethasone, granisetron and so on. Fosaprepitant has been evaluated to use in the prevention of chemotherapy-induced nausea and vomiting (CINV) by combination with a 5-HT3 antagonist and a steroid [1].
References:
[1] Van Belle SJ1, Cocquyt V. Fosaprepitant dimeglumine (MK-0517 or L-785,298), an intravenous neurokinin-1 antagonist for the prevention of chemotherapy induced nausea and vomiting.Expert Opin Pharmacother. 2008 Dec; 9(18):3261-70.
- Clemastanin A
Catalog No.:BCC8151
CAS No.:172670-47-2
- Eucalyptone
Catalog No.:BCN1111
CAS No.:172617-99-1
- Murrayamine E
Catalog No.:BCN7908
CAS No.:172617-68-4
- Naringin 4'-glucoside
Catalog No.:BCN8196
CAS No.:17257-21-5
- Senkyunolide R
Catalog No.:BCC9144
CAS No.:172549-37-0
- Eupatarone
Catalog No.:BCN7199
CAS No.:17249-61-5
- 9-Deacetyl-9-benzoyl-10-debenzoyltaxchinin A
Catalog No.:BCN7671
CAS No.:172486-22-5
- 3',4',5',3,5,6,7-Heptamethoxyflavone
Catalog No.:BCN1110
CAS No.:17245-30-6
- Coumurrayin
Catalog No.:BCN1109
CAS No.:17245-25-9
- 2-(Hydroxymethyl)anthraquinone
Catalog No.:BCN3090
CAS No.:17241-59-7
- 2-Hydroxy-3-methylanthraquinone
Catalog No.:BCN1108
CAS No.:17241-40-6
- Dihydrodaidzein
Catalog No.:BCN2819
CAS No.:17238-05-0
- Fmoc-β-Homo-Val-OH
Catalog No.:BCC2626
CAS No.:172695-33-9
- Senkyunolide S
Catalog No.:BCC9145
CAS No.:172723-28-3
- Varespladib (LY315920)
Catalog No.:BCC2310
CAS No.:172732-68-2
- Mudanpioside C
Catalog No.:BCN2798
CAS No.:172760-03-1
- Isocucurbitacin B
Catalog No.:BCN8101
CAS No.:17278-28-3
- H-Aib-OEt.HCl
Catalog No.:BCC2670
CAS No.:17288-15-2
- PP 1
Catalog No.:BCC3630
CAS No.:172889-26-8
- PP 2 (AG 1879)
Catalog No.:BCC3631
CAS No.:172889-27-9
- LY 272015 hydrochloride
Catalog No.:BCC7558
CAS No.:172895-15-7
- LY 266097 hydrochloride
Catalog No.:BCC7856
CAS No.:172895-39-5
- Isosinensetin
Catalog No.:BCN2919
CAS No.:17290-70-9
- PB 28 dihydrochloride
Catalog No.:BCC7411
CAS No.:172907-03-8
Systemic hypersensitivity to fosaprepitant - A report of two cases.[Pubmed:27872331]
J Oncol Pharm Pract. 2018 Jan;24(1):76-78.
Fosaprepitant is a widely administered antiemetic used mainly for moderately to highly emetogenic chemotherapy. Local injection site reactions are the most common type of infusion reaction reported from Fosaprepitant. At our institution, two separate patients have experienced systemic hypersensitivity reactions to their infusions of Fosaprepitant. We report a review of the literature and the details of these reactions.
Growth inhibition of formed corneal neovascularization following Fosaprepitant treatment.[Pubmed:28205389]
Acta Ophthalmol. 2017 Nov;95(7):e641-e648.
PURPOSE: The aim of this study was to test the efficacy of Neurokinin-1 Receptor (NK-1R) antagonist -Fosaprepitant- in inducing regression of established corneal neovascularization (CNV). METHODS: Twenty C57BL/6 mice underwent alkali burn. Seven days later, when corneal neovessels had developed, they received Fosaprepitant 10 mg/ml, administered topically six times a day in the right eye for 10 days. In parallel, a group of 20 causticated mice was treated with normal saline, as control. A second independent experiment was also performed (n = 10/group). Finally, ten healthy mice received the same topical treatment for 10 days to evaluate Fosaprepitant safety. Haemangiogenesis and lymphangiogenesis were measured by means of vesselj plugin (imagej). Secondary endpoints, such as leucocyte infiltration, corneal opacity and corneal fluorescein staining were also evaluated. Inflammatory cell composition was assessed by flow cytometry. Differences between groups were assessed using unpaired t-test, Mann-Whitney U-test or two-way anova, as appropriate. RESULTS: Topical Fosaprepitant administration induced a significant reduction of (i) CD31(+) blood corneal neovessels (-27%, p = 0.0132), (ii) LYVE1(+) lymphatic corneal neovessels (-31%, p = 0.0118) and (iii) CD45(+) leucocyte infiltration (-36%; p = 0.0237). The second independent experiment confirmed these data. Moreover, Fosaprepitant-treated corneas showed a reduction in opacity, no impairment in corneal fluorescein staining and decreased infiltration of neutrophils (-72%, p < 0.05) and macrophages (-75%, p < 0.01). Finally, topical Fosaprepitant was not toxic to the ocular surface: no signs of conjunctivitis, opacity, perforations or corneal fluorescein staining were detected. Similarly, corneal TUJ1(+) nerve density was not affected. CONCLUSIONS: Our data suggest that NK-1R antagonists, such as Fosaprepitant, could be a new, promising therapeutic tool to inhibit CNV after this has been established.
Role of fosaprepitant, a neurokinin Type 1 receptor antagonist, in morphine-induced antinociception in rats.[Pubmed:27756950]
Indian J Pharmacol. 2016 Jul-Aug;48(4):394-398.
OBJECTIVES: Opioids such as morphine form the cornerstone in the treatment of moderate to severe pain. However, opioids also produce serious side effects such as tolerance. Fosaprepitant is a substance P (SP) receptor antagonist, which is used for treating chemotherapy-induced nausea and vomiting. SP is an important neuropeptide mediating transmission of pain at the spinal level. Thus, it was hypothesized that combining morphine with Fosaprepitant would increase the antinociceptive effect of morphine. The objectives were to evaluate the effect of Fosaprepitant on morphine-induced antinociception in rats and to investigate its mechanism of action. METHODS: Sprague-Dawley rats were injected with morphine (10 mg/kg twice daily) and/or Fosaprepitant (30 mg/kg once daily) for 7 days. Pain threshold was assessed by the hot plate test. Expression of SP and calcitonin gene-related peptide (CGRP) in the spinal cords of these rats was evaluated by immunohistochemistry. RESULTS: Morphine administration resulted in an antinociceptive effect compared to the control group (day 1 and to a lesser extent on day 4). The decreased antinociception despite continued morphine treatment indicated development of tolerance. Co-administration of Fosaprepitant attenuated tolerance to morphine (days 1 and 3) and increased the antinociceptive effect compared to control group (days 1-4). Expression of SP was increased in the morphine + Fosaprepitant group. CONCLUSIONS: The results show that Fosaprepitant attenuates the development of tolerance to morphine and thereby, increases the antinociceptive effect. This is likely linked to decreased release of SP from presynaptic terminals.
Fosaprepitant versus droperidol for prevention of PONV in craniotomy: a randomized double-blind study.[Pubmed:27757553]
J Anesth. 2017 Feb;31(1):82-88.
PURPOSE: Postoperative nausea and vomiting (PONV) is a common complication after craniotomy. Vomiting may be a potentially hazardous complication in neurosurgical patients. We compared the efficacy of Fosaprepitant and droperidol for the prevention of PONV, vomiting in particular, after craniotomy. METHODS: Patients scheduled to undergo elective craniotomy were enrolled in the study and randomly divided in a double-blind manner into two groups to receive either 150 mg of Fosaprepitant (group F) or 1.25 mg of droperidol (group D). Dexamethasone (9.9 mg) was given to all patients, except those with diabetes mellitus. The incidence of PONV, frequency of vomiting, nausea score, and use of rescue antiemetic during the first 72 h after surgery were assessed at five time intervals (0-2, 2-6, 6-24, 24-48, and 48-72 h). RESULTS: Of the 200 randomized patients eligible for entry into the study, 186 were ultimately included for analysis. There were no significant differences in demographics or intraoperative variables between the two treatment groups. Over the entire 72-h post-craniotomy observation period the overall and cumulative incidence of vomiting was significantly lower in group F patients than in group D patients, while there were no between-group differences in the overall and cumulative incidence of PONV or in complete response (no PONV and no rescue antiemetic). The incidence and frequency of vomiting during each of the five observational periods were significantly lower in group F patients than group D patients, although there were no differences in the nausea score and antiemetic use between the groups. CONCLUSION: Based on the results, Fosaprepitant was more effective than droperidol in the prevention of vomiting after craniotomy over the entire 72-h study period. However, there was no difference in the incidence of nausea and antiemetic use.


