IsokaempferideCAS# 1592-70-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1592-70-7 | SDF | Download SDF |
| PubChem ID | 5280862 | Appearance | Yellow powder |
| Formula | C16H12O6 | M.Wt | 300.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
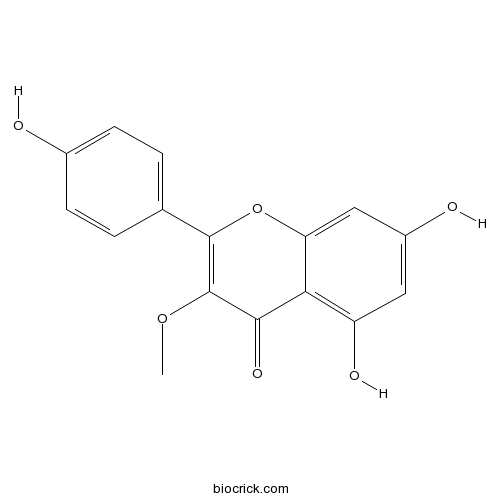
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-methoxychromen-4-one | ||
| SMILES | COC1=C(OC2=CC(=CC(=C2C1=O)O)O)C3=CC=C(C=C3)O | ||
| Standard InChIKey | VJJZJBUCDWKPLC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O6/c1-21-16-14(20)13-11(19)6-10(18)7-12(13)22-15(16)8-2-4-9(17)5-3-8/h2-7,17-19H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isokaempferide shows anti-inflammatory effects, the anti-inflammatory effects can be explained, at least in part, by reducing neutrophil degranulation, myeloperoxidase activity, mediators as well as TNF-alpha secretion. 2. Isokaempferide is used as a bronchodilator, can induce relaxation of guinea-pig isolated trachea. 3. The hydroxyflavones (luteolin, apigenin and isokaempferide) exert comparable antiproliferative activities against malignant and normal cells. 4. Isokaempferide shows strong inhibitory effect on TNF-alpha-induced liver cell death with the IC50 value of 22.8 microM, suggests that it has hepatoprotective activity. |
| Targets | TNF-α | PGE | Calcium Channel | ATPase | Potassium Channel |

Isokaempferide Dilution Calculator

Isokaempferide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-NIO dihydrochloride
Catalog No.:BCC6689
CAS No.:159190-44-0
- MM 77 dihydrochloride
Catalog No.:BCC6854
CAS No.:159187-70-9
- L-755,507
Catalog No.:BCC7282
CAS No.:159182-43-1
- CARIPORIDE
Catalog No.:BCC6432
CAS No.:159138-80-4
- 3F8
Catalog No.:BCC6112
CAS No.:159109-11-2
- F1839-I
Catalog No.:BCN6450
CAS No.:159096-49-8
- 6-Benzyloxyindole
Catalog No.:BCC8769
CAS No.:15903-94-3
- Wedelobatin B
Catalog No.:BCN6730
CAS No.:1589488-35-6
- Wedelobatin A
Catalog No.:BCN6731
CAS No.:1589488-34-5
- Secoisolarisiresinol Diglucoside
Catalog No.:BCC9140
CAS No.:158932-33-3
- Boc-D-Tryptophanol
Catalog No.:BCC2698
CAS No.:158932-00-4
- APC 366
Catalog No.:BCC7392
CAS No.:158921-85-8
- PNU 22394 hydrochloride
Catalog No.:BCC7285
CAS No.:15923-42-9
- MPDC
Catalog No.:BCC6873
CAS No.:159262-32-5
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- N-Acetyl-O-phosphono-Tyr-Glu-Glu-Ile-Glu
Catalog No.:BCC5853
CAS No.:159439-02-8
- Enfuvirtide
Catalog No.:BCC5641
CAS No.:159519-65-0
- Fluconazole mesylate
Catalog No.:BCC4236
CAS No.:159532-41-9
- GR 55562 dihydrochloride
Catalog No.:BCC6913
CAS No.:159533-25-2
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- 3,4-Secotirucalla-4(28,7,24-triene-3),26-dioic acid
Catalog No.:BCN1549
CAS No.:159623-48-0
- Saropyrone
Catalog No.:BCN7692
CAS No.:159650-12-1
- Wikstrol A
Catalog No.:BCN7938
CAS No.:159736-35-3
- ISRIB (trans-isomer)
Catalog No.:BCC5340
CAS No.:1597403-47-8
Hepatoprotective effect of Combretum quadrangulare and its constituents.[Pubmed:10784427]
Biol Pharm Bull. 2000 Apr;23(4):456-60.
The MeOH extract of leaves of Combretum quadrangulare showed significant hepatoprotective effect on D-galactosamine (D-GalN)/lipopolysaccharide (LPS)-induced experimental liver injury in mice and on D-GalN/tumor necrosis factor-alpha (TNF-alpha)-induced cell death in primary cultured mouse hepatocytes. Phytochemical investigation led to the isolation of thirty cycloartane-type triterpenes together with betulinic acid, beta-sitosterol, beta-sitosterol glucoside, 4 flavones (34-37), and 3 flavone C-glucosides (38-40). These compounds showed various potencies of hepatoprotective effect on D-GalN/TNF-alpha-induced cell death in primary cultured mouse hepatocytes. Quadrangularol B (29), methyl quadrangularate I (33), kamatakenin (34), 5,7,4'-trihydroxy-3,3'-dimethoxyflavone (35), 5,4'-dihydroxy-3,7,3'-trimethoxyflavone (36) and Isokaempferide (37) showed strong inhibitory effect on TNF-alpha-induced cell death with IC50 values of 34.3, 33.7, 13.3, 22.4, 13.4 and 22.8 microM, respectively, whereas clinically-used silibinin had an IC50 value of 39.6 microM and glycyrrhizin showed very weak inhibitory effect. Methyl quadrangularates A (30) and N (32), norquadrangularic acid B (31) and vitexin (40) also showed potent inhibition on TNF-alpha-induced cell death with IC50 values of 45.7, 89.3, 67.6 and 40.1 microM, respectively. The flavonoids and some of the cycloartane-type triterpenes appeared to be the hepatoprotective principles of the leaves of C. quadrangulare.
Mechanisms underlying the relaxation induced by isokaempferide from Amburana cearensis in the guinea-pig isolated trachea.[Pubmed:16455108]
Life Sci. 2006 May 30;79(1):98-104.
The present study examines possible mechanisms by which the flavonoid Isokaempferide (IKPF; 5,7,4'-trihydroxy-3-methoxyflavone) from Amburana cearensis, a Brazilian medicinal plant popularly used as bronchodilator, induces relaxation of guinea-pig isolated trachea. In the trachea (with intact epithelium) contracted by carbachol, IKPF (1-1000 microM) caused a graded relaxation, and the epithelium removal increased the sensitivity of the airway smooth muscle to IKPF (EC50, in intact tissue: 77.4 [54.8-109.2] microM; in denuded epithelium: 15.0 [11.3-20.1] microM). The IKPF-induced relaxation was inhibited in 41% by the nitric oxide (NO) synthase inhibitor L-NAME (100 microM); in 31% and 50% by the soluble guanylate cyclase (sGC) inhibitor ODQ (3 and 33 microM); by propranolol (31%) and also by capsaicin (37%). In the trachea pre-contracted by 40 mM KCl the pre-incubation with glibenclamide (33 microM) or iberiotoxin (IbTX, 0.1 microM), selective K(+) channel inhibitors, inhibited the IKPF-induced relaxation by 39% and 38%, respectively. On the other hand, 4-aminopyridine (100 microM), a nonselective K(+) channel antagonist, did not significantly influence the effect of IKPF, while IbTX induced a rightward displacement of the IKPF concentration-response curve. However, in muscle pre-contracted with 120 mM KCl the relaxant effect of IKPF was significantly reduced and not affected by glibenclamide. In conclusion, these results indicate a direct and epithelium-independent relaxant effect of IKPF on smooth muscle fibers. Although this IKPF relaxant action seems to be multi-mediated, it occurs via both Ca(2+) and ATP-sensitive K(+) channels, but some other possible mechanisms unrelated to K(+) channels cannot be excluded.
Effects of amburoside A and isokaempferide, polyphenols from Amburana cearensis, on rodent inflammatory processes and myeloperoxidase activity in human neutrophils.[Pubmed:19053991]
Basic Clin Pharmacol Toxicol. 2009 Mar;104(3):198-205.
The present study evaluated the anti-inflammatory activity of amburoside A (a phenol glucoside) and Isokaempferide (a flavonol) isolated from the trunk bark of Amburana cearensis, a medicinal plant used in northeast Brazil for the treatment of asthma. Animals (male Wistar rats or Swiss mice) pre-treated with amburoside A (25 and 50 mg/kg) or Isokaempferide (12.5, 25 and 50 mg/kg), orally or intraperitoneally, showed a significant inhibition of the paw oedema induced by carrageenan (1%), prostaglandin E(2) (30 nmol/paw), histamine (200 microg/paw) or serotonin (200 microg/paw). Histological and morphometric evaluations of the rat paw oedema induced by carrageenan showed that amburoside A and Isokaempferide also inhibited the accumulation of inflammatory cells. Amburoside A reduced significantly the paw oedema and the increase in vascular permeability induced by dextran, as related to the control group. Similar results were observed with the Isokaempferide pre-treatment. Furthermore, amburoside A or Isokaempferide inhibited both leucocyte and neutrophil migrations, in mouse peritoneal cavity, after the carrageenan injection. The polyphenols were not cytotoxic and blocked N-formyl-methyl-leucyl-phenylalanine-induced myeloperoxidase release and activity in human neutrophils. In addition, amburoside A and Isokaempferide at 50 and 100 microg/ml concentrations reduced significantly the lipopolysaccharide-mediated increase in tumour necrosis factor-alpha (TNF-alpha) levels. These results provide, for the first time, evidence to support the anti-inflammatory activity of amburoside A and Isokaempferide that seems to be related to an inhibition of inflammatory mediators, such as TNF-alpha, as well as histamine, serotonin and prostaglandin E(2), besides leucocyte infiltration in a dose- or concentration-dependent manner. These anti-inflammatory effects can be explained, at least in part, by the ability of these compounds to reduce neutrophil degranulation, myeloperoxidase activity, mediators as well as TNF-alpha secretion.
Antiproliferative activity of flavonoids: influence of the sequential methoxylation state of the flavonoid structure.[Pubmed:22184071]
Phytother Res. 2012 Jul;26(7):1023-8.
Dracocephalum kotschyi Boiss. has been used as part of an ethnobotanical remedy against many forms of human cancer in Iran. It has been demonstrated that a flavonoid named xanthomicrol from D. kotschyi contributes to its preferential antiproliferative activity against malignant cells. In the present study, the antiproliferative activity of its flavonoid fraction was further characterized. Using liquid-liquid extraction and a semi-preparative reversed-phase HPLC method, eight flavonoid aglycones were isolated from the aerial parts of the plant and their identities were confirmed through MS and NMR analyses as luteolin, naringenin, apigenin, Isokaempferide, cirsimaritin, penduletin, xanthomicrol and calycopterin. The in vitro antiproliferative activity of each compound was evaluated against a panel of established normal and malignant cell lines using the MTT assay and some structure-activity relationships were observed. The hydroxyflavones (luteolin, apigenin and Isokaempferide) exerted comparable antiproliferative activities against malignant and normal cells, while the methoxylated hydroxyflavones (cirsimaritin, penduletin, xanthomicrol and calycopterin) showed preferential activities against tumor cells. This activity may be of value in treating tumors as it would exert few side effects in normal tissues. Xanthomicrol selectively inhibited the growth of human gastric adenocarcinoma, while calycopterin selectively prevented human acute promyelocytic leukemia and human colon carcinoma cells proliferation.


