Fluconazole mesylateCAS# 159532-41-9 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 159532-41-9 | SDF | Download SDF |
| PubChem ID | 17785855 | Appearance | Powder |
| Formula | C14H16F2N6O4S | M.Wt | 402.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
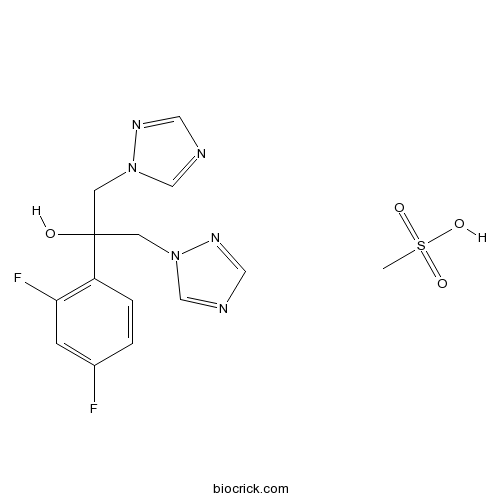
| Chemical Name | 2-(2,4-difluorophenyl)-1,3-bis(1,2,4-triazol-1-yl)propan-2-ol;methanesulfonic acid | ||
| SMILES | CS(=O)(=O)O.C1=CC(=C(C=C1F)F)C(CN2C=NC=N2)(CN3C=NC=N3)O | ||
| Standard InChIKey | DWJUZHMCPBPNFH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H12F2N6O.CH4O3S/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21;1-5(2,3)4/h1-3,6-9,22H,4-5H2;1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fluconazole mesylate Dilution Calculator

Fluconazole mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4852 mL | 12.4261 mL | 24.8521 mL | 49.7043 mL | 62.1303 mL |
| 5 mM | 0.497 mL | 2.4852 mL | 4.9704 mL | 9.9409 mL | 12.4261 mL |
| 10 mM | 0.2485 mL | 1.2426 mL | 2.4852 mL | 4.9704 mL | 6.213 mL |
| 50 mM | 0.0497 mL | 0.2485 mL | 0.497 mL | 0.9941 mL | 1.2426 mL |
| 100 mM | 0.0249 mL | 0.1243 mL | 0.2485 mL | 0.497 mL | 0.6213 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fluconazole (mesylate) is a triazole antifungal drug used in the treatment and prevention of superficial and systemic fungal infections.
- Enfuvirtide
Catalog No.:BCC5641
CAS No.:159519-65-0
- N-Acetyl-O-phosphono-Tyr-Glu-Glu-Ile-Glu
Catalog No.:BCC5853
CAS No.:159439-02-8
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- MPDC
Catalog No.:BCC6873
CAS No.:159262-32-5
- PNU 22394 hydrochloride
Catalog No.:BCC7285
CAS No.:15923-42-9
- Isokaempferide
Catalog No.:BCN3790
CAS No.:1592-70-7
- L-NIO dihydrochloride
Catalog No.:BCC6689
CAS No.:159190-44-0
- MM 77 dihydrochloride
Catalog No.:BCC6854
CAS No.:159187-70-9
- L-755,507
Catalog No.:BCC7282
CAS No.:159182-43-1
- CARIPORIDE
Catalog No.:BCC6432
CAS No.:159138-80-4
- 3F8
Catalog No.:BCC6112
CAS No.:159109-11-2
- F1839-I
Catalog No.:BCN6450
CAS No.:159096-49-8
- GR 55562 dihydrochloride
Catalog No.:BCC6913
CAS No.:159533-25-2
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- 3,4-Secotirucalla-4(28,7,24-triene-3),26-dioic acid
Catalog No.:BCN1549
CAS No.:159623-48-0
- Saropyrone
Catalog No.:BCN7692
CAS No.:159650-12-1
- Wikstrol A
Catalog No.:BCN7938
CAS No.:159736-35-3
- ISRIB (trans-isomer)
Catalog No.:BCC5340
CAS No.:1597403-47-8
- Ibutamoren Mesylate
Catalog No.:BCC1638
CAS No.:159752-10-0
- Fmoc-Lys(Ac)-OH
Catalog No.:BCC3514
CAS No.:159766-56-0
- Ulipristal
Catalog No.:BCC4944
CAS No.:159811-51-5
- Sikokianin C
Catalog No.:BCN6827
CAS No.:159813-69-1
- 4'-O-Methylirenolone
Catalog No.:BCN7174
CAS No.:159853-36-8
- Mc-Val-Cit-PABC-PNP
Catalog No.:BCC4028
CAS No.:159857-81-5
Gateways to clinical trials.[Pubmed:16636723]
Methods Find Exp Clin Pharmacol. 2006 Mar;28(2):121-42.
Gateways to Clinical Trials are a guide to the most recent clinical trials in current literature and congresses. The data in the following tables have been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: 131I-labetuzumab; Abacavir sulfate, abatacept, adalimumab, ademetionine, adjuvanted influenza vaccine, alefacept, alemtuzumab, amlodipine, amphotericin B, anakinra, aripiprazole, aspirin, axitinib; Betamethasone dipropionate, bevacizumab, biphasic insulin aspart, bortezomib, bosentan, botulinum toxin type B, BQ-123; Calcium folinate, canertinib dihydrochloride, carboplatin, carmustine, cetirizine hydrochloride, cetuximab, cholecalciferol, ciclesonide, ciclosporin, cinacalcet hydrochloride, cisplatin, clarithromycin, clofazimine, cold-adapted influenza vaccine trivalent, CpG-7909; Darbepoetin alfa, darifenacin hydrobromide, DB-289, desloratadine, Dexamet, dicycloverine hydrochloride, dimethyl fumarate, docetaxel, dolastatin 10, drospirenone, drospirenone/estradiol, duloxetine hydrochloride; Ecogramostim, edotecarin, efaproxiral sodium, enalapril maleate, epoetin beta, epoprostenol sodium, epratuzumab, erlotinib hydrochloride, escitalopram oxalate, estradiol, etanercept; Fluconazole, fludarabine phosphate, fluorouracil; Gefitinib, gemcitabine, Ghrelin (human), glibenclamide, glimepiride, GTI-2040; Haloperidol, human insulin, hydrocortisone probutate; Imatinib mesylate, indisulam, influenza vaccine, inhaled insulin, insulin aspart, insulin glulisine, insulin lispro, irinotecan, ispronicline; Lamivudine, lamivudine/zidovudine/abacavir sulfate, lapatinib, letrozole, levocetirizine, lomustine, lonafarnib, lumiracoxib;Magnesium sulfate, MD-1100, melphalan, metformin hydrochloride, methotrexate, metoclopramide hydrochloride, mitiglinide calcium hydrate, monophosphoryl lipid A, montelukast sodium, motexafin gadolinium, mycophenolate mofetil, mycophenolic acid sodium salt; Nitisinone; Omalizumab, omapatrilat, ONYX-015, oxaliplatin; Paclitaxel, paclitaxel nanoparticles, panitumumab, parathyroid hormone (human recombinant), peginterferon alfa-2a, peginterferon alfa-2b, peginterferon alfa-2b/ribavirin, pertuzumab, phosphatidylcholine-rich phospholipid mixture, pimecrolimus, pioglitazone hydrochloride, pramlintide acetate, prasterone; QR-333; Ranelic acid distrontium salt, ranolazine, rasagiline mesilate, RFB4(dsFv)-PE38, ribavirin, rifabutin, risperidone, rituximab, rofecoxib, rosiglitazone maleate, rosiglitazone maleate/metformin hydrochloride, rotavirus vaccine; S-236, salmeterol xinafoate, sarizotan hydrochloride, sildenafil, sildenafil citrate, sunitinib malate; Tadalafil, tegaserod maleate, temozolomide, tenofovir disoproxil fumarate, teriparatide, tiotropium bromide, tipifarnib, trabectedin, treprostinil sodium; Vandetanib, vardenafil hydrochloride hydrate, vatalanib succinate, vinflunine, virosome influenza vaccine, voriconazole; Zidovudine.
Role of DNA mismatch repair and double-strand break repair in genome stability and antifungal drug resistance in Candida albicans.[Pubmed:17965250]
Eukaryot Cell. 2007 Dec;6(12):2194-205.
Drug resistance has become a major problem in the treatment of Candida albicans infections. Genome changes, such as aneuploidy, translocations, loss of heterozygosity, or point mutations, are often observed in clinical isolates that have become resistant to antifungal drugs. To determine whether these types of alterations result when DNA repair pathways are eliminated, we constructed yeast strains bearing deletions in six genes involved in mismatch repair (MSH2 and PMS1) or double-strand break repair (MRE11, RAD50, RAD52, and YKU80). We show that the mre11Delta/mre11Delta, rad50Delta/rad50Delta, and rad52Delta/rad52Delta mutants are slow growing and exhibit a wrinkly colony phenotype and that cultures of these mutants contain abundant elongated pseudohypha-like cells. These same mutants are susceptible to hydrogen peroxide, tetrabutyl hydrogen peroxide, UV radiation, camptothecin, ethylmethane sulfonate, and methylmethane sulfonate. The msh2Delta/msh2Delta, pms1Delta/pms1Delta, and yku80Delta/yku80Delta mutants exhibit none of these phenotypes. We observed an increase in genome instability in mre11Delta/mre11Delta and rad50Delta/rad50Delta mutants by using a GAL1/URA3 marker system to monitor the integrity of chromosome 1. We investigated the acquisition of drug resistance in the DNA repair mutants and found that deletion of mre11Delta/mre11Delta, rad50Delta/rad50Delta, or rad52Delta/rad52Delta leads to an increased susceptibility to fluconazole. Interestingly, we also observed an elevated frequency of appearance of drug-resistant colonies for both msh2Delta/msh2Delta and pms1Delta/pms1Delta (MMR mutants) and rad50Delta/rad50Delta (DSBR mutant). Our data demonstrate that defects in double-strand break repair lead to an increase in genome instability, while drug resistance arises more rapidly in C. albicans strains lacking mismatch repair proteins or proteins central to double-strand break repair.
The role of Candida albicans homologous recombination factors Rad54 and Rdh54 in DNA damage sensitivity.[Pubmed:21951709]
BMC Microbiol. 2011 Sep 27;11:214.
BACKGROUND: The fungal pathogen Candida albicans is frequently seen in immune suppressed patients, and resistance to one of the most widely used antifungals, fluconazole (FLC), can evolve rapidly. In recent years it has become clear that plasticity of the Candida albicans genome contributes to drug resistance through loss of heterozygosity (LOH) at resistance genes and gross chromosomal rearrangements that amplify gene copy number of resistance associated genes. This study addresses the role of the homologous recombination factors Rad54 and Rdh54 in cell growth, DNA damage and FLC resistance in Candida albicans. RESULTS: The data presented here support a role for homologous recombination in cell growth and DNA damage sensitivity, as Candida albicans rad54Delta/rad54Delta mutants were hypersensitive to MMS and menadione, and had an aberrant cell and nuclear morphology. The Candida albicans rad54Delta/rad54Delta mutant was defective in invasion of Spider agar, presumably due to the altered cellular morphology. In contrast, mutation of the related gene RDH54 did not contribute significantly to DNA damage resistance and cell growth, and deletion of either Candida albicans RAD54 or Candida albicans RDH54 did not alter FLC susceptibility. CONCLUSIONS: Together, these results support a role for homologous recombination in genome stability under nondamaging conditions. The nuclear morphology defects in the rad54Delta/rad54Delta mutants show that Rad54 performs an essential role during mitotic growth and that in its absence, cells arrest in G2. The viability of the single mutant rad54Delta/rad54Delta and the inability to construct the double mutant rad54Delta/rad54Delta rdh54Delta/rdh54Delta suggests that Rdh54 can partially compensate for Rad54 during mitotic growth.
Impact of imatinib on the pharmacokinetics and in vivo efficacy of etoposide and/or ifosfamide.[Pubmed:17963518]
BMC Pharmacol. 2007 Oct 27;7:13.
BACKGROUND: Using a human small cell lung cancer (SCLC) xenografted in nude mice, we have previously reported enhanced tumor growth inhibition following chemotherapy in combination with imatinib (STI571). We therefore investigated the in vivo impact of imatinib on the pharmacokinetics and efficacy of chemotherapy. METHODS: Two different human tumors were used: SCLC6 small cell lung cancer xenografted in nude mice, and LY-3 EBV-associated human B-cell lymphoma xenografted in SCID mice. Plasma, urine, and fecal concentrations of etoposide (VP16) were determined by a validated high performance liquid chromatography method. Plasma concentrations of ifosfamidewere determined by a validated gas chromatography assay with nitrogen-phosphorus detection. RESULTS: Slight tumor growth inhibition was induced by imatinib administered alone in one in vivo EBV-associated B-cell lymphomatous xenograft. In contrast, an increase of the chemotherapy-induced antitumor effect was observed in the lymphoma model but not in a small cell lung cancer model when mice bearing human xenografted tumors were treated concomitantly by imatinib and chemotherapy. This antitumor effect was not influenced by concomitant administration of fluconazole. The AUC0-3 h (Area Under the concentration-time Curve) of etoposide was increased when mice were treated with etoposide + imatinib due to decreased fecal excretion. In contrast, imatinib did not appear to influence the urinary excretion of etoposide, and concomitant administration of the CYP3A4 inhibitor, fluconazole, with imatinib did not modify the pharmacokinetics of etoposide plus imatinib alone. CONCLUSION: Altogether, these results therefore justify further prospective phase I and II clinical trials with combinations of etoposide-based chemotherapy and imatinib in patients with certain cancers, such as malignant lymphoma, with careful toxicologic monitoring.
1-n-Hexadecyl-3-methylimidazolium methanesulfonate and chloride salts with effective activities against Candida tropicalis biofilms.[Pubmed:26331427]
Lett Appl Microbiol. 2015 Nov;61(5):504-10.
UNLABELLED: Although the use of catheters in critically ill patients is mostly inevitable, this invasive procedure comes together with several health risks. Within this context, the contamination with Candida tropicalis is a primary concern as this highly prevalent pathogenic yeast can develop an extensive polymeric matrix that hinders the drugs' penetration and its effective treatment. This study addresses the potential for the 1-n-hexadecyl-3-methylimidazolium methanesulfonate (C16 MImMeS) and chloride (C16 MImCl) salts for eliminating the viable cells of biofilms of Candida tropicalis, compared to the performance of chlorhexidine (CHX) and fluconazole (FLZ). The minimum concentration required of C16 MImMeS, C16 MImCl, CHX and FLZ for elimination of the biofilm's viable cells (MBEC) was evaluated through microtitre plate biofilm exposure with different concentrations of these substances. These concentrations were determined at 80% of effective activity against the biofilm's viable cells by using the MTT reduction assay. C16 MImMeS and C16 MImCl were able to eliminate the viable cells at much lower concentrations (15.6 and 0.45 mug ml(-1) respectively) than CHX (1250 mug ml(-1) ) and FLZ (resistance of the viable cells). This demonstrates the high potential of these substances for nosocomial infections control. SIGNIFICANCE AND IMPACT OF THE STUDY: The 1-n-hexadecyl-3-methylimidazolium methanesulfonate (C16 MImMeS) and chloride (C16 MImCl) salts are extremely effective in eliminating the viable cells of Candida tropicalis biofilms, which allows the use of much lower concentrations than with the antimicrobial of choice (chlorhexidine) in hospital practices. These findings indicate these imidazolium salts as high-potential candidates for asepsis of medical environments and materials, including implants.


