CARIPORIDEPotent NHE inhibitor CAS# 159138-80-4 |
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- Epoxomicin
Catalog No.:BCC1235
CAS No.:134381-21-8
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 159138-80-4 | SDF | Download SDF |
| PubChem ID | 151172 | Appearance | Powder |
| Formula | C12H17N3O3S | M.Wt | 283.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | HOE 642 | ||
| Solubility | DMSO : ≥ 100 mg/mL (352.92 mM) *"≥" means soluble, but saturation unknown. | ||
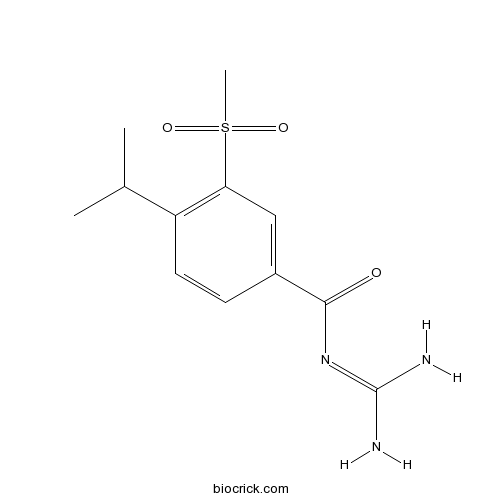
| Chemical Name | N-(diaminomethylidene)-3-methylsulfonyl-4-propan-2-ylbenzamide | ||
| SMILES | CC(C)C1=C(C=C(C=C1)C(=O)N=C(N)N)S(=O)(=O)C | ||
| Standard InChIKey | IWXNYAIICFKCTM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H17N3O3S/c1-7(2)9-5-4-8(11(16)15-12(13)14)6-10(9)19(3,17)18/h4-7H,1-3H3,(H4,13,14,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective Na+/H+ exchanger isoform 1 (NHE1) inhibitor (IC50 values are 0.05, 3 and 1000 μM for NHE1, NHE3 and NHE2 respectively). Attenuates ischemia-induced cardiomyocyte apoptosis in vitro. Reduces cardiac arrhythmia in vivo. Also promotes apoptosis in cancer cells overexpressing NHE1. Orally active. |

CARIPORIDE Dilution Calculator

CARIPORIDE Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5292 mL | 17.646 mL | 35.292 mL | 70.5841 mL | 88.2301 mL |
| 5 mM | 0.7058 mL | 3.5292 mL | 7.0584 mL | 14.1168 mL | 17.646 mL |
| 10 mM | 0.3529 mL | 1.7646 mL | 3.5292 mL | 7.0584 mL | 8.823 mL |
| 50 mM | 0.0706 mL | 0.3529 mL | 0.7058 mL | 1.4117 mL | 1.7646 mL |
| 100 mM | 0.0353 mL | 0.1765 mL | 0.3529 mL | 0.7058 mL | 0.8823 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description:
IC50: 0.05, 3 and 1000 μM for NHE1, NHE3 and NHE2, respectively
Na–H exchange (NHE) represents an important mechanism for mediating such injury. NHE represents an important mechanism for the development of myocardial ischemic and reperfusion injury and inhibitors have been consistently shown to protect the ischemic and reperfused heart by correcting the ionic imbalance associated with this form of pathological insult. Cariporide is a potent NHE inhibitor.
In vitro: Cariporide concentration dependently inhibited the amiloride sensitive sodium influx in rabbit erythrocytes, reduced the swelling of human platelets caused by intracellular acidification, and delayed pH recovery in rat cardiomyocytes [1].
In vivo: In anaesthetised rats undergoing coronary artery ligation intravenous and oral pretreatment with Cariporide caused a dose dependent reduction or a complete prevention of ventricular premature beats, ventricular fibrillation, and ventricular tachycardia. The compound was well tolerated and neutral to circulatory variables [1].
Clinical trial: Cariporide has been evaluated in a large dose-finding Phase II/Phase III clinical trial to assess the efficacy in patients with acute coronary syndromes. Overall results failed to demonstrate protection but sub-group analysis revealed significant risk reductions with the highest cariporide dose especially in high risk patients undergoing coronary artery bypass surgery [2].
Reference:
[1] Scholz W, Albus U, Counillon L, G?gelein H, Lang HJ, Linz W, Weichert A, Sch?lkens BA. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res. 1995 Feb;29(2):260-8.
[2] Karmazyn M. Pharmacology and clinical assessment of cariporide for the treatment coronary artery diseases. Expert Opin Investig Drugs. 2000 May;9(5):1099-108.
- 3F8
Catalog No.:BCC6112
CAS No.:159109-11-2
- F1839-I
Catalog No.:BCN6450
CAS No.:159096-49-8
- 6-Benzyloxyindole
Catalog No.:BCC8769
CAS No.:15903-94-3
- Wedelobatin B
Catalog No.:BCN6730
CAS No.:1589488-35-6
- Wedelobatin A
Catalog No.:BCN6731
CAS No.:1589488-34-5
- Secoisolarisiresinol Diglucoside
Catalog No.:BCC9140
CAS No.:158932-33-3
- Boc-D-Tryptophanol
Catalog No.:BCC2698
CAS No.:158932-00-4
- APC 366
Catalog No.:BCC7392
CAS No.:158921-85-8
- GR 231118
Catalog No.:BCC7085
CAS No.:158859-98-4
- BET-BAY 002
Catalog No.:BCC5510
CAS No.:1588521-78-1
- ent-17-Hydroxykaura-9(11),15-dien-19-oic acid
Catalog No.:BCN6788
CAS No.:1588516-88-4
- 3Alaph-Tigloyloxypterokaurene L3
Catalog No.:BCN6787
CAS No.:1588516-87-3
- L-755,507
Catalog No.:BCC7282
CAS No.:159182-43-1
- MM 77 dihydrochloride
Catalog No.:BCC6854
CAS No.:159187-70-9
- L-NIO dihydrochloride
Catalog No.:BCC6689
CAS No.:159190-44-0
- Isokaempferide
Catalog No.:BCN3790
CAS No.:1592-70-7
- PNU 22394 hydrochloride
Catalog No.:BCC7285
CAS No.:15923-42-9
- MPDC
Catalog No.:BCC6873
CAS No.:159262-32-5
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- N-Acetyl-O-phosphono-Tyr-Glu-Glu-Ile-Glu
Catalog No.:BCC5853
CAS No.:159439-02-8
- Enfuvirtide
Catalog No.:BCC5641
CAS No.:159519-65-0
- Fluconazole mesylate
Catalog No.:BCC4236
CAS No.:159532-41-9
- GR 55562 dihydrochloride
Catalog No.:BCC6913
CAS No.:159533-25-2
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
Cardio-protective signalling by glyceryl trinitrate and cariporide in a model of donor heart preservation.[Pubmed:25459486]
Heart Lung Circ. 2015 Mar;24(3):306-18.
BACKGROUND: Storage of donor hearts in cardioplegic solutions supplemented with agents that mimic the ischaemic preconditioning response enhanced their post-reperfusion function. The present study examines the minimisation of cell death and activation of pro-survival signalling directed towards maintenance of mitochondrial homeostasis in hearts arrested and stored in two such agents, glyceryl-trinitrate, a nitric oxide donor and CARIPORIDE, (a sodium-hydrogen exchange inhibitor). METHODS: After baseline functional measurement, isolated working rat hearts were arrested and stored for 6h at 4 degrees C in either Celsior((R)), Celsior((R)) containing 0.1mg/ml glyceryl-trinitrate, 10muM CARIPORIDE or both agents. After reperfusion, function was remeasured. Hearts were then processed for immunoblotting or histology. RESULTS: Necrotic and apoptotic markers present in the Celsior((R)) group post-reperfusion were abolished by glyceryl-trinitrate, CARIPORIDE or both. Increased phosphorylation of ERK and Bcl2, after reperfusion in groups stored in glyceryl-trinitrate, CARIPORIDE or both along with increased phospho-STAT3 levels in the glyceryl-trinitrate/CARIPORIDE group correlated with functional recovery. Inhibition of STAT3 phosphorylation blocked recovery. No phospho-Akt increase was seen in any treatment. CONCLUSIONS: Activation of signalling pathways that favour mitophagy activation (ERK and Bcl2 phosphorylation) and maintenance of mitochondrial transition pore closure after reperfusion (STAT3 and ERK phosphorylation) were crucial for functional recovery of the donor heart.
[Cariporide Pretreatment Attenuates Lung Ischemia-Reperfusion Injury in Rabbits].[Pubmed:26121860]
Sichuan Da Xue Xue Bao Yi Xue Ban. 2015 May;46(3):394-8.
OBJECTIVE: To explore the effects of selective Na+/H+ exchanger antagonist CARIPORIDE on ischemia-reperfusion induced lung injury. METHODS: Twenty four New Zealand White rabbits with lung ischemia-reperfusion model were established and randomly divided into four groups (n=6 per group) including sham group (S group), ischemia-reperfusion group (IR group), low potassium dextran group (LPD group) and CARIPORIDE group (HOE group). Blood and lung tissue samples were obtained for blood gas, biochemical analyses and histologic examination. RESULTS: Systemic administration of HOE increased oxygenation index (arterial oxygen tension/fraction of inspire oxygen, PaO2/FiO2) and superoxide dismutase (SOD) activities, while decreased malondialdehyde (MDA) contents, proinflammatory cytokines and natrium hydrogen exchanger-1 (NHEI) expressions, along with the reduction of lung water content (LWC) except for histologic evaluation scores (P< 0. 05, versus IR group and LPD group). CONCLUSION: Systemic administration of CARIPORIDE before ischemia could protects the lung from ischemia-reperfusion injury via decreasing NHE1 expression. The protective effect seems to be closely related to regulating intracellular calcium overload, oxidative damage and antioxidant enzyme activities and neutrophil infiltration.
Na(+)/H(+) exchanger in the regulation of platelet activation and paradoxical effects of cariporide.[Pubmed:25595121]
Exp Neurol. 2015 Oct;272:11-6.
Platelets are anucleated cell fragments derived from mature megakaryocytes and function in hemostasis when the endothelium is injured. Hemostasis involving platelets can be divided into four phases: adhesion, activation, secretion, and aggregation. Platelet activation requires a rise in intracellular Ca(2+) concentrations and results in both a morphological change and the secretion of platelet granule contents. Na(+)/H(+) exchanger isoform 1 (NHE1) regulates the intracellular pH (pHi) and the volume of platelets. In addition, NHE1 plays a large role in platelet activation. Thrombus generation involves NHE1 activation and an increase in [Ca(2+)]i, which results from NHE1-mediated Na(+) overload and the reversal of the Na(+)/Ca(2+) exchanger. CARIPORIDE (HOE-642), a potent NHE1 inhibitor, has inhibitory effects on the degranulation of human platelets, the formation of platelet-leukocyte-aggregates, and the activation of the GPIIb/IIIa receptor (PAC-1). However, despite the demonstrated protection against myocardial infarction as mediated by CARIPORIDE in patients undergoing coronary artery bypass graft surgery, the EXPEDITION clinical trial revealed that CARIPORIDE treatment increased mortality due to thromboembolic stroke. These findings suggest that a better understanding of NHE1 and its effect on platelet function and procoagulant factor regulation is warranted in order to develop therapies using NHE inhibitors.
Effects of intravenous cariporide on release of norepinephrine and myoglobin during myocardial ischemia/reperfusion in rabbits.[Pubmed:25139834]
Life Sci. 2014 Oct 2;114(2):102-6.
AIMS: To examine the effects of CARIPORIDE, a Na(+)/H(+) exchanger-1 inhibitor, on cardiac norepinephrine (NE) and myoglobin release during myocardial ischemia/reperfusion by applying a microdialysis technique to the rabbit heart. MAIN METHODS: In anesthetized rabbits, two dialysis probes were implanted into the left ventricular myocardium and were perfused with Ringer's solution. CARIPORIDE (0.3mg/kg) was injected intravenously, followed by occlusion of the left circumflex coronary artery. During 30-min coronary occlusion followed by 30-min reperfusion, four consecutive 15-min dialysate samples (two during ischemia and two during reperfusion) were collected in vehicle and CARIPORIDE-treated groups. Dialysate myoglobin and NE concentrations were measured by immunochemistry and high-performance liquid chromatography, respectively. KEY FINDINGS: Dialysate myoglobin and NE concentrations increased significantly during myocardial ischemia/reperfusion in both vehicle and CARIPORIDE-treated groups (P<0.01 vs. baseline). In CARIPORIDE-treated group, dialysate myoglobin concentrations were significantly lower than those in vehicle group throughout ischemia/reperfusion (P<0.01 at 0-15 min of ischemia, P<0.05 at 15-30 min of ischemia, P<0.01 at 0-15 min of reperfusion, and P<0.01 at 15-30 min of reperfusion). However, dialysate NE concentrations in CARIPORIDE-treated group were lower than those in vehicle group only during ischemia (P<0.01 at 0-15 min of ischemia, and P<0.05 at 15-30 min of ischemia). SIGNIFICANCE: When administered before ischemia, CARIPORIDE reduces myoglobin release during ischemia/reperfusion and decreases NE release during ischemia.
Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway.[Pubmed:14568900]
Circulation. 2003 Nov 4;108(18):2275-81.
BACKGROUND: The Na+-H+ exchanger figures prominently in cardiac ischemia-reperfusion injury. Several experimental and clinical studies have demonstrated a cardioprotective effect of Na+-H+ exchanger inhibition; however, the precise mechanisms have not been established. METHODS AND RESULTS: We examined the effects of CARIPORIDE (HOE642, 10 micromol/L) on cell death induced by oxidative stress (H2O2, 100 micromol/L) in cultured neonatal rat cardiomyocytes. CARIPORIDE significantly suppressed markers of cell death, such as TUNEL positivity and caspase-3 cleavage, at 8 or 16 hours after H2O2. The early phase of cell death, reported by increases in phosphatidylserine exposure and propidium iodide uptake, was also inhibited by CARIPORIDE. To explore the mechanisms of cell protection, we examined the effects of CARIPORIDE on increases in intracellular Na+ and Ca2+ induced by oxidative stress. CARIPORIDE remarkably suppressed cytosolic Na+ and Ca2+ accumulation. Next, we investigated the effects of CARIPORIDE on mitochondria-associated death process. Mitochondrial Ca2+ overload induced by H2O2 was remarkably suppressed by CARIPORIDE. Loss of mitochondrial membrane potential is a critical step of the death pathway; CARIPORIDE prevented mitochondrial membrane potential loss induced by H2O2. CONCLUSIONS: CARIPORIDE protects cardiomyocytes against oxidant-induced cell death by preserving intracellular ion homeostasis and mitochondrial integrity.
A rapid ischemia-induced apoptosis in isolated rat hearts and its attenuation by the sodium-hydrogen exchange inhibitor HOE 642 (cariporide).[Pubmed:9405190]
J Mol Cell Cardiol. 1997 Nov;29(11):3169-74.
Apoptosis is a potentially important myocardial response to pathology including ischemia and reperfusion. Na-H exchange (NHE) represents an important mechanism for mediating such injury. The present study was done to determine if NHE inhibition can affect early apoptosis in an acute model of ischemia and reperfusion. Isolated rat hearts were subjected to zero-flow ischemia for various durations with or without subsequent 30 min of reperfusion. Nick-end-labelling of biotin-dUTP (TUNEL staining), as well as DNA extraction followed by agarose gel electrophoresis, were used to semiquantify apoptotic cells and identify DNA laddering, respectively. Apoptosis first appeared after 10 min of ischemia and reached a maximum level after 30 min. The number of apoptotic cells after 30 min of ischemia was 31 +/- 3 per 100 high power microscopic fields, whereas in reperfused hearts the number of cells was 34 +/- 3. To determine the effect of NHE inhibition, hearts were pretreated 15 min prior to ischemia with HOE 642, a potent and specific inhibitor of the isoform (NHE-1) found in myocardium. HOE 642 significantly reduced the number of apoptotic cells in the ischemic and reperfused heart to 2 +/- 1 and 6 +/- 1, respectively (P<0.05 from untreated hearts). DNA laddering was not observed with electrophoretic DNA analysis, likely owing to the small number of apoptotic cells involved. Hearts recovered nearly 100% of function in both groups, although there was a significantly higher recovery after 1 and 2 min of reperfusion in those hearts treated with HOE 642. Our study shows that apoptosis, albeit very mild in nature, can be rapidly induced in isolated hearts by a relatively brief period of ischemia without reperfusion, which can be markedly attenuated by the NHE inhibitor HOE 642. The ability of HOE 642 to markedly attenuate apoptosis may be important in terms of understanding the drug's cardioprotective properties as well as the overall role of NHE in heart disease.
Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion.[Pubmed:7736504]
Cardiovasc Res. 1995 Feb;29(2):260-8.
OBJECTIVE: The aim was to characterise the new compound HOE642 as a selective and cardioprotective Na+/H+ exchange inhibitor in various models. METHODS: The effect of HOE642 was tested in the osmotically activated Na+/H+ exchange of rabbit erythrocytes and in propionate induced swelling of human thrombocytes. Recovery of pH after an NH4Cl prepulse and effects on other ion transport systems by patch clamp technique were investigated in rat cardiomyocytes. NHE subtype specifity of the compound was determined by 22Na+ uptake inhibition in a fibroblast cell line separately expressing subtype isoforms 1-3. Protective effects of HOE642 in cardiac ischaemia and reperfusion by ligation of coronary artery were investigated in isolated working rat hearts and in anaesthetised rats. RESULTS: HOE642 concentration dependently inhibited the amiloride sensitive sodium influx in rabbit erythrocytes, reduced the swelling of human platelets induced by intracellular acidification, and delayed pH recovery in rat cardiomyocytes. In the isolated working rat heart subjected to ischaemia and reperfusion HOE642 dose dependently reduced the incidence and the duration of reperfusion arrhythmias. It also reduced the the release of lactate dehydrogenase and creatine kinase, and preserved the tissue content of glycogen, ATP, and creatine phosphate. In anaesthetised rats undergoing coronary artery ligation intravenous and oral pretreatment with HOE642 caused a dose dependent reduction or a complete prevention of ventricular premature beats, ventricular tachycardia, and ventricular fibrillation. The compound was well tolerated and neutral to circulatory variables. Other cardiovascular agents tested in this model were not, or were only partly, effective at doses showing marked cardiodepressive effects. CONCLUSIONS: HOE642 is a very selective NHE subtype 1 inhibitor showing cardioprotective and antiarrhythmic effects in ischaemic and reperfused hearts. Further development of well tolerated compounds like HOE642 could lead to a new therapeutic approach in clinical indications related to cardiac ischaemia and reperfusion.


