L-755,507β3 adrenergic receptor agonist CAS# 159182-43-1 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 159182-43-1 | SDF | Download SDF |
| PubChem ID | 9829836 | Appearance | Powder |
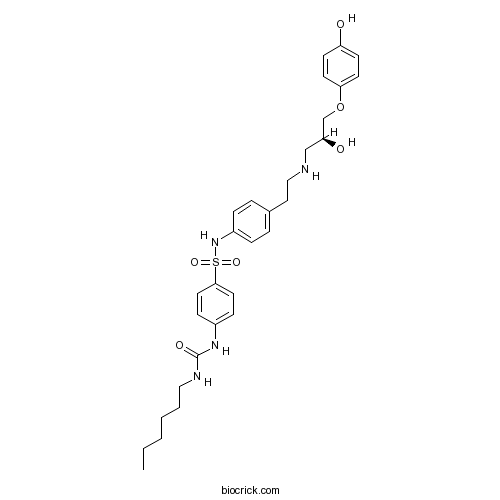
| Formula | C30H40N4O6S | M.Wt | 584.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (171.02 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-hexyl-3-[4-[[4-[2-[[(2S)-2-hydroxy-3-(4-hydroxyphenoxy)propyl]amino]ethyl]phenyl]sulfamoyl]phenyl]urea | ||
| SMILES | CCCCCCNC(=O)NC1=CC=C(C=C1)S(=O)(=O)NC2=CC=C(C=C2)CCNCC(COC3=CC=C(C=C3)O)O | ||
| Standard InChIKey | NYYJKMXNVNFOFQ-MHZLTWQESA-N | ||
| Standard InChI | InChI=1S/C30H40N4O6S/c1-2-3-4-5-19-32-30(37)33-24-10-16-29(17-11-24)41(38,39)34-25-8-6-23(7-9-25)18-20-31-21-27(36)22-40-28-14-12-26(35)13-15-28/h6-17,27,31,34-36H,2-5,18-22H2,1H3,(H2,32,33,37)/t27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent β3-adrenergic receptor partial agonist > 1000-fold selective over β1- and β2-adrenoceptors (EC50 values are 0.43, 580 and > 10000 nM for activation of cloned human β3-, β1- and β2-adrenoceptors respectively). Stimulates lipolysis in rhesus adipocytes in vitro (EC50 = 3.9 nM). Enhances CRISPR-mediated homology-directed repair (HDR) efficiency 2-3-fold for large fragments and ~9-fold for point mutations, in human induced pluripotent stem cells (iPSCs). |

L-755,507 Dilution Calculator

L-755,507 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7102 mL | 8.551 mL | 17.1019 mL | 34.2038 mL | 42.7548 mL |
| 5 mM | 0.342 mL | 1.7102 mL | 3.4204 mL | 6.8408 mL | 8.551 mL |
| 10 mM | 0.171 mL | 0.8551 mL | 1.7102 mL | 3.4204 mL | 4.2755 mL |
| 50 mM | 0.0342 mL | 0.171 mL | 0.342 mL | 0.6841 mL | 0.8551 mL |
| 100 mM | 0.0171 mL | 0.0855 mL | 0.171 mL | 0.342 mL | 0.4275 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 13 nM (binding at the human β3 adrenergic receptor) [1]
Benzenesulfonamide derivative L-755,507 is a partial agonist for the human β3 receptor, with maximal activation 52% of that evoked by isoproterenol [2]. β3 adrenergic receptor is a G protein-coupled receptors which can enhancement of lipolysis in adipose tissue.
In vitro: L-755,507 displays an excellent activity profile as an extremely potent human β3 adrenergic receptor agonist (β3 EC50 0.43 nM), with >440-fold selectivity over β1 and β2 binding [1]. L-755,507 is also a potent and selective b3 partial agonist in rhesus monkeys as assessed by its affinity for the cloned b adrenergic receptors, and stimulates lipolysis in rhesus adipocytes with an EC50 = 3.9 nM [2].
In vivo: Dose rhesus monkeys with L-755,507 elicits lipolysis and metabolic rate elevation. The ED50 for glycerolemia was 0.03 mg/kg and the ED50 for tachycardia was 2.5 mg/kg, and stimulates metabolic rate by ~ 30% after acute bolus intravenous administration of 0.1 mg/kg [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Parmee ER, Ok HO, Candelore MR, Tota L, Deng L, Strader CD, Wyvratt MJ, Fisher MH, Weber AE. Discovery of L-755,507: a subnanomolar human beta 3 adrenergic receptor agonist. Bioorg Med Chem Lett. 1998 May 5;8(9):1107-12.
[2] Fisher MH, Amend AM, Bach TJ, Barker JM, Brady EJ, Candelore MR, Carroll D, Cascieri MA, Chiu SH, Deng L, Forrest MJ, Hegarty-Friscino B, Guan XM, Hom GJ, Hutchins JE, Kelly LJ, Mathvink RJ, Metzger JM, Miller RR, Ok HO, Parmee ER, Saperstein R, Strader CD, Stearns RA, MacIntyre DE, et al. A selective human beta3 adrenergic receptor agonist increases metabolic rate in rhesus monkeys. J Clin Invest. 1998 Jun 1;101(11):2387-93.
- CARIPORIDE
Catalog No.:BCC6432
CAS No.:159138-80-4
- 3F8
Catalog No.:BCC6112
CAS No.:159109-11-2
- F1839-I
Catalog No.:BCN6450
CAS No.:159096-49-8
- 6-Benzyloxyindole
Catalog No.:BCC8769
CAS No.:15903-94-3
- Wedelobatin B
Catalog No.:BCN6730
CAS No.:1589488-35-6
- Wedelobatin A
Catalog No.:BCN6731
CAS No.:1589488-34-5
- Secoisolarisiresinol Diglucoside
Catalog No.:BCC9140
CAS No.:158932-33-3
- Boc-D-Tryptophanol
Catalog No.:BCC2698
CAS No.:158932-00-4
- APC 366
Catalog No.:BCC7392
CAS No.:158921-85-8
- GR 231118
Catalog No.:BCC7085
CAS No.:158859-98-4
- BET-BAY 002
Catalog No.:BCC5510
CAS No.:1588521-78-1
- ent-17-Hydroxykaura-9(11),15-dien-19-oic acid
Catalog No.:BCN6788
CAS No.:1588516-88-4
- MM 77 dihydrochloride
Catalog No.:BCC6854
CAS No.:159187-70-9
- L-NIO dihydrochloride
Catalog No.:BCC6689
CAS No.:159190-44-0
- Isokaempferide
Catalog No.:BCN3790
CAS No.:1592-70-7
- PNU 22394 hydrochloride
Catalog No.:BCC7285
CAS No.:15923-42-9
- MPDC
Catalog No.:BCC6873
CAS No.:159262-32-5
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- N-Acetyl-O-phosphono-Tyr-Glu-Glu-Ile-Glu
Catalog No.:BCC5853
CAS No.:159439-02-8
- Enfuvirtide
Catalog No.:BCC5641
CAS No.:159519-65-0
- Fluconazole mesylate
Catalog No.:BCC4236
CAS No.:159532-41-9
- GR 55562 dihydrochloride
Catalog No.:BCC6913
CAS No.:159533-25-2
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- 3,4-Secotirucalla-4(28,7,24-triene-3),26-dioic acid
Catalog No.:BCN1549
CAS No.:159623-48-0
Discovery of L-755,507: a subnanomolar human beta 3 adrenergic receptor agonist.[Pubmed:9871717]
Bioorg Med Chem Lett. 1998 May 5;8(9):1107-12.
A study of 4-acylaminobenzenesulfonamides in a cloned human beta 3 adrenergic receptor assay resulted in the discovery of n-hexylurea, L-755,507 (22). This 0.43 nM beta 3 agonist, which is > 440-fold selective over both beta 1 and beta 2 binding, is among the most potent human beta 3 agonists reported to date.
Small molecules enhance CRISPR genome editing in pluripotent stem cells.[Pubmed:25658371]
Cell Stem Cell. 2015 Feb 5;16(2):142-7.
The bacterial CRISPR-Cas9 system has emerged as an effective tool for sequence-specific gene knockout through non-homologous end joining (NHEJ), but it remains inefficient for precise editing of genome sequences. Here we develop a reporter-based screening approach for high-throughput identification of chemical compounds that can modulate precise genome editing through homology-directed repair (HDR). Using our screening method, we have identified small molecules that can enhance CRISPR-mediated HDR efficiency, 3-fold for large fragment insertions and 9-fold for point mutations. Interestingly, we have also observed that a small molecule that inhibits HDR can enhance frame shift insertion and deletion (indel) mutations mediated by NHEJ. The identified small molecules function robustly in diverse cell types with minimal toxicity. The use of small molecules provides a simple and effective strategy to enhance precise genome engineering applications and facilitates the study of DNA repair mechanisms in mammalian cells.
A selective human beta3 adrenergic receptor agonist increases metabolic rate in rhesus monkeys.[Pubmed:9616210]
J Clin Invest. 1998 Jun 1;101(11):2387-93.
Activation of beta3 adrenergic receptors on the surface of adipocytes leads to increases in intracellular cAMP and stimulation of lipolysis. In brown adipose tissue, this serves to up-regulate and activate the mitochondrial uncoupling protein 1, which mediates a proton conductance pathway that uncouples oxidative phosphorylation, leading to a net increase in energy expenditure. While chronic treatment with beta3 agonists in nonprimate species leads to uncoupling protein 1 up-regulation and weight loss, the relevance of this mechanism to energy metabolism in primates, which have much lower levels of brown adipose tissue, has been questioned. With the discovery of L-755,507, a potent and selective partial agonist for both human and rhesus beta3 receptors, we now demonstrate that acute exposure of rhesus monkeys to a beta3 agonist elicits lipolysis and metabolic rate elevation, and that chronic exposure increases uncoupling protein 1 expression in rhesus brown adipose tissue. These data suggest a role for beta3 agonists in the treatment of human obesity.


