L-690,330competitive inhibitor of inositol monophosphatase (IMPase) CAS# 142523-38-4 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 142523-38-4 | SDF | Download SDF |
| PubChem ID | 132449 | Appearance | Powder |
| Formula | C8H12O8P2 | M.Wt | 298.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water with gentle warming | ||
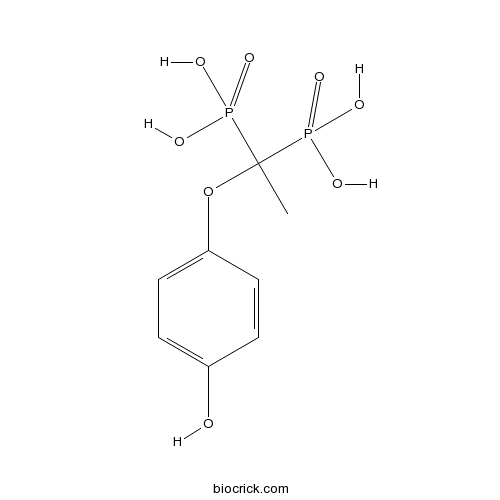
| Chemical Name | [1-(4-hydroxyphenoxy)-1-phosphonoethyl]phosphonic acid | ||
| SMILES | CC(OC1=CC=C(C=C1)O)(P(=O)(O)O)P(=O)(O)O | ||
| Standard InChIKey | JKOCAAWWDVHWKB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H12O8P2/c1-8(17(10,11)12,18(13,14)15)16-7-4-2-6(9)3-5-7/h2-5,9H,1H3,(H2,10,11,12)(H2,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A potent inhibitor of inositol monophophatase; stable to hydrolysis. Induces autophagy in COS-7 cells independently of mTOR inhibition. |

L-690,330 Dilution Calculator

L-690,330 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3542 mL | 16.7712 mL | 33.5424 mL | 67.0848 mL | 83.856 mL |
| 5 mM | 0.6708 mL | 3.3542 mL | 6.7085 mL | 13.417 mL | 16.7712 mL |
| 10 mM | 0.3354 mL | 1.6771 mL | 3.3542 mL | 6.7085 mL | 8.3856 mL |
| 50 mM | 0.0671 mL | 0.3354 mL | 0.6708 mL | 1.3417 mL | 1.6771 mL |
| 100 mM | 0.0335 mL | 0.1677 mL | 0.3354 mL | 0.6708 mL | 0.8386 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
L-690,330 is a competitive inhibitor of inositol monophosphatase (IMPase) with a Ki value of between 0.2 and 2 μM depending on the source of IMPase [1].
In the phosphatidylinositol (PI) cycle cell signalling pathway, IMPase is involved as a key enzyme. Inositol monophosphates, inositol(1)phosphate [Ins(l)P], inositol(3)phosphate [Ins(3)P], and inositol(4)phosphate [Ins(4)P] are hydrolyzed by IMPase and produce free inositol. Free inositol can then be used to synthesize PI [1].
In carbachol-stimulated m1 CHO cells, the treatment with L-690,330 alone did not increase the amount of [3H]InsP1. In the absence of L-690,330, the treatment with carbachol alone increased levels of [3H]InsP1, resulted in levels ranging from 20 to 100% above basal [3H]InsP1 levels. In the presence of carbachol, lithium at 10 mM increased the amount of [3H]InsP1 by approximate five folds (relative to [3H]InsP1 levels in carbachol-stimulated cells) and the EC50 value of this effect was about 1 mM. In carbachol-stimulated cells, L-690,330 at 10 mM resulted in only ~40% of the maximum response seen in the case with lithium treatment [1].
In mouse brains, in the absence of L-690,330, pilocarpine produced approximately doubled levels of Ins(1)P. In the absence of pilocarpine, L-690,330 did not affect Ins(1)P levels. Under pilocarpine-stimulated conditions, treatment with L-690,330 resulted in a pronounced accumulation of Ins(1)P, with the maximum level observed 1 h after injection. The maximum was of an approximate four-fold elevation [1].
Reference:
[1]. Atack JR, Cook SM, Watt AP, et al. In Vitro and In Vivo Inhibition of Inositol Monophosphatase by the Bisphosphonate L-690,330. Journal of neurochemistry, 1993, 60(2): 652-658.
- L-690,488
Catalog No.:BCC5667
CAS No.:142523-14-6
- MK-5172 sodium salt
Catalog No.:BCC1765
CAS No.:1425038-27-2
- 19-Nortestosterone acetate
Catalog No.:BCC8445
CAS No.:1425-10-1
- Glyasperin A
Catalog No.:BCN6228
CAS No.:142474-52-0
- 3-O-beta-D-apiofuranosyl(1-2)-beta-D-glucopyranosyl rhamnocitrin 4-O-beta-D-glucopyranoside
Catalog No.:BCN8141
CAS No.:142473-99-2
- Amthamine dihydrobromide
Catalog No.:BCC6744
CAS No.:142457-00-9
- Lobetyolinin
Catalog No.:BCN3322
CAS No.:142451-48-7
- Myricetin 3-O-beta-D-xylopyranosyl(1-2)-beta-D-glucopyranoside
Catalog No.:BCN8140
CAS No.:142449-93-2
- NHS-SS-Biotin
Catalog No.:BCC3581
CAS No.:142439-92-7
- Crovatin
Catalog No.:BCN2517
CAS No.:142409-09-4
- Mesterolone
Catalog No.:BCC9023
CAS No.:1424-00-6
- Didemethylpseudoaspidin AA
Catalog No.:BCN3777
CAS No.:142382-28-3
- 1,2,3,4,7-Pentamethoxy-9H-xanthen-9-one
Catalog No.:BCN1570
CAS No.:14254-96-7
- Cimidahurinine
Catalog No.:BCN6229
CAS No.:142542-89-0
- Sageone
Catalog No.:BCN3144
CAS No.:142546-15-4
- A 484954
Catalog No.:BCC6203
CAS No.:142557-61-7
- Glyasperin D
Catalog No.:BCN6836
CAS No.:142561-10-2
- Calanolide E
Catalog No.:BCN6230
CAS No.:142566-61-8
- Asperuloside
Catalog No.:BCN6231
CAS No.:14259-45-1
- Narirutin
Catalog No.:BCN6300
CAS No.:14259-46-2
- Didymin
Catalog No.:BCN3327
CAS No.:14259-47-3
- Deacetylasperulosidic acid
Catalog No.:BCN3323
CAS No.:14259-55-3
- Daphylloside
Catalog No.:BCN6232
CAS No.:14260-99-2
- Macrocarpal C
Catalog No.:BCN6233
CAS No.:142628-53-3
Effects of L-690,488, a prodrug of the bisphosphonate inositol monophosphatase inhibitor L-690,330, on phosphatidylinositol cycle markers.[Pubmed:8035344]
J Pharmacol Exp Ther. 1994 Jul;270(1):70-6.
In order to enhance the entry into cells of L-690,330, a bisphosphonate inhibitor of inositol monophosphatase (IMPase; a key, enzyme in the phosphatidylinositol (Pl) cell signaling pathway), the tetrapivaloyloxymethyl ester prodrug, L-690,488 [tetrapivaloyloxymethyl 1-(4-hydroxyphenoxy)ethane-1,1-bisphosphonate], was synthesized. The effects of L-690,488 were studied in cholinergically (carbachol)-stimulated rat cortical slices and Chinese hamster ovary cells stably transfected with the human muscarinic m1 receptor (m1 CHO cells). The accumulation of [3H]inositol monophosphates or [3H]cytidine monophosphorylphosphatidate ([3H]CMP-PA) after [3H]inositol or [3H]cytidine prelabeling, respectively, and inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass were measured. In rat cortical slices and m1 CHO cells, the maximum response and time course of accumulation of [3H]inositol monophosphates for L-690,488 and lithium were similar. However, the concentrations of L-690,488 required to produce these effects (EC50 values of 3.7 +/- 0.9 and 1.0 +/- 0.2 microM in cortical slices and m1 CHO cells, respectively) were much lower than with lithium (0.3-1.5 mM). Likewise, the time course and maximum accumulation of [3H] CMP-PA in L-690,488-treated m1 CHO cells was similar to lithium but L-690,488 was again much more potent (EC50 values = 3.5 +/- 0.3 microM and 0.52 +/- 0.03 mM for L-690,488 and lithium, respectively). In addition, L-690,488 attenuated the carbachol-induced elevation of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate in m1 CHO cells, an effect reported previously with lithium. These results are all consistent with L-690,488 and lithium both depleting intracellular inositol as a consequence of inhibition of IMPase. That these effects of L-690,488 on the PI cycle are indeed due to inositol depletion is shown by the observation that the effects of L-690,488 on CMP-PA accumulation could be overcome by addition of exogenous myo-inositol (EC50 = 1.7 +/- 0.5 mM). These data show that inhibition of IMPase produces effects on the PI cycle comparable to lithium. As a corollary, the effects of lithium on the PI cycle are therefore consistent with its major mechanism of action being inhibition of IMPase.
The inositol monophosphatase inhibitor L-690,330 affects pilocarpine-behavior and the forced swim test.[Pubmed:23344554]
Psychopharmacology (Berl). 2013 Jun;227(3):503-8.
RATIONALE: Lithium has been a standard pharmacological treatment for bipolar disorder over the last 60 years; however, the molecular targets through which lithium exerts its therapeutic effects are still not defined. Attenuation of the phosphatidylinositol signal transduction pathway as a consequence of inhibition of inositol monophosphatase (IMPase) has been proposed as one of the possible mechanisms for lithium-induced mood stabilization. OBJECTIVES: The objective was to study the behavioral effect of the specific competitive IMPase inhibitor L-690,330 in mice in the lithium-sensitive pilocarpine-induced seizures paradigm and the forced swim test (FST). METHODS: The inhibitor was administered intracerebroventricularly in liposomes. RESULTS: L-690,330 increased the sensitivity to subconvulsive doses of pilocarpine and decreased immobility time in the FST. CONCLUSIONS: It is possible that the behavioral effects of lithium in the pilocarpine-induced seizures and in the FST are mediated through the inhibition of IMPase, but reversal of the inhibitor's effect with intracerebroventricular inositol would be an important further step in proof.
In vitro and in vivo inhibition of inositol monophosphatase by the bisphosphonate L-690,330.[Pubmed:8380439]
J Neurochem. 1993 Feb;60(2):652-8.
We have previously described the synthesis of bisphosphonate-containing inhibitors of inositol monophosphatase. In the present study, a more detailed examination of the in vitro and in vivo properties of one of these compounds, L-690,330, is described. L-690,330 is a competitive inhibitor of inositol monophosphatase with a Ki, depending on the source of IMPase, of between 0.2 and 2 microM. Although approximately 1,000-fold more potent in vitro than lithium, in muscarinic ml receptor-transfected Chinese hamster ovary cells prelabelled with [3H]inositol, L-690,330 only produced 40% of the accumulation of [3H]inositol monophosphates achieved by lithium at the same concentration (10 mM), suggesting that the ability of L-690,330 to cross the cell membrane is limited. Nevertheless, under conditions of cholinergic stimulation (100 mg/kg of pilocarpine s.c.), high doses of L-690,330 were able to increase brain inositol(l)phosphate levels in vivo to three- to fourfold control levels. This effect was dose dependent (ED50 = 0.3 mmol/kg s.c.) and was maximal after 1 h. In peripheral tissues, the effects of L-690,330 on inositol(l)phosphate levels mimicked those of lithium both qualitatively and quantitatively. However, in the brain, the effects of L-690,330 were much less than seen with lithium, consistent with the blood-brain barrier restricting access of the polar L-690,330 into the CNS, thereby further limiting entry of compound into cells in the brain. In the future, it may be possible to develop prodrugs of this compound, which circumvent many of the cell permeability problems inherent in bisphosphonate compounds.
Chemical modulators of autophagy as biological probes and potential therapeutics.[Pubmed:21164513]
Nat Chem Biol. 2011 Jan;7(1):9-17.
Autophagy is an evolutionarily conserved mechanism for protein degradation that is critical for the maintenance of homeostasis in man. Autophagy has unexpected pleiotropic functions that favor survival of the cell, including nutrient supply under starvation, cleaning of the cellular interior, defense against infection and antigen presentation. Moreover, defective autophagy is associated with a diverse range of disease states, including neurodegeneration, cancer and Crohn's disease. Here we discuss the roles of mammalian autophagy in health and disease and highlight recent advances in pharmacological manipulation of autophagic pathways as a therapeutic strategy for a variety of pathological conditions.
Lithium induces autophagy by inhibiting inositol monophosphatase.[Pubmed:16186256]
J Cell Biol. 2005 Sep 26;170(7):1101-11.
Macroautophagy is a key pathway for the clearance of aggregate-prone cytosolic proteins. Currently, the only suitable pharmacologic strategy for up-regulating autophagy in mammalian cells is to use rapamycin, which inhibits the mammalian target of rapamycin (mTOR), a negative regulator of autophagy. Here we describe a novel mTOR-independent pathway that regulates autophagy. We show that lithium induces autophagy, and thereby, enhances the clearance of autophagy substrates, like mutant huntingtin and alpha-synucleins. This effect is not mediated by glycogen synthase kinase 3beta inhibition. The autophagy-enhancing properties of lithium were mediated by inhibition of inositol monophosphatase and led to free inositol depletion. This, in turn, decreased myo-inositol-1,4,5-triphosphate (IP3) levels. Our data suggest that the autophagy effect is mediated at the level of (or downstream of) lowered IP3, because it was abrogated by pharmacologic treatments that increased IP3. This novel pharmacologic strategy for autophagy induction is independent of mTOR, and may help treatment of neurodegenerative diseases, like Huntington's disease, where the toxic protein is an autophagy substrate.


