LIMKi 3LIMK2 inhibitor CAS# 1338247-35-0 |
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

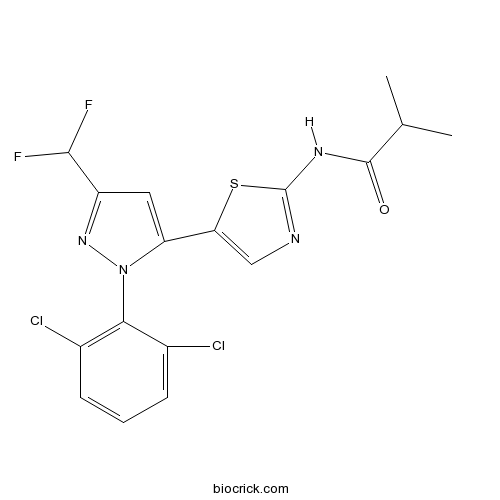
| Cas No. | 1338247-35-0 | SDF | Download SDF |
| PubChem ID | 56965901 | Appearance | Powder |
| Formula | C17H14Cl2F2N4OS | M.Wt | 431.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMS 5 | ||
| Solubility | DMSO : ≥ 34 mg/mL (78.83 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[5-[2-(2,6-dichlorophenyl)-5-(difluoromethyl)pyrazol-3-yl]-1,3-thiazol-2-yl]-2-methylpropanamide | ||
| SMILES | CC(C)C(=O)NC1=NC=C(S1)C2=CC(=NN2C3=C(C=CC=C3Cl)Cl)C(F)F | ||
| Standard InChIKey | IVUGBSGLHRJSSP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14Cl2F2N4OS/c1-8(2)16(26)23-17-22-7-13(27-17)12-6-11(15(20)21)24-25(12)14-9(18)4-3-5-10(14)19/h3-8,15H,1-2H3,(H,22,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent LIM kinase inhibitor (IC50 values are 7 and 8 nM for LIMK1 and LIMK2 respectively). Inhibits cofilin phosphorylation in MDA-MB-231 breast cancer cells. Reduces MDA-MB-231 tumor cell invasion in a 3D matrigel invasion assay. |

LIMKi 3 Dilution Calculator

LIMKi 3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3186 mL | 11.5931 mL | 23.1863 mL | 46.3725 mL | 57.9656 mL |
| 5 mM | 0.4637 mL | 2.3186 mL | 4.6373 mL | 9.2745 mL | 11.5931 mL |
| 10 mM | 0.2319 mL | 1.1593 mL | 2.3186 mL | 4.6373 mL | 5.7966 mL |
| 50 mM | 0.0464 mL | 0.2319 mL | 0.4637 mL | 0.9275 mL | 1.1593 mL |
| 100 mM | 0.0232 mL | 0.1159 mL | 0.2319 mL | 0.4637 mL | 0.5797 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 8 nmol/L for LIMK2
LIMKi 3 is a novel LIMK2 inhibitor.
LIM kinases (LIMKs) are key cell cytoskeleton regulators playing a prominent role in cancer manifestation and neuronal diseases. The LIMK family has been found to consist of two homologues, LIMK1 and LIMK2, which differ from one another in their intercellular localizations, expression profiles, and functions. Moreover, LIMKs also play a contributory role in several neuro-developmental disorders as well as in cancer growth and metastasis.
In vitro: Previous study showed that LIMKi 3 inhibited LIMK2 with high specificity, and showed little or no cross-reactivity with LIMK1. It was also found that LIMKi 3 could decrease phosphorylated cofilin (p-cofilin) levels and therefore inhibited growth of several cancer cell lines including pancreatic cancer, schwannoma and glioma [1].
In vivo: In a nude mouse Panc-1 xenograft model, LIMKi 3 was found to be able to reduce the tumor size and p-cofilin levels in the Panc-1 tumors, which led to propose LIMKi 3 as a candidate drug for cancer therapy [1].
Clinical trial: N/A
Reference:
[1] Rak R,Haklai R,Elad-Tzfadia G,Wolfson HJ,Carmeli S,Kloog Y. Novel LIMK2 Inhibitor Blocks Panc-1 Tumor Growth in a mouse xenograft model. Oncoscience.2014 Jan 1;1(1):39-48.
- Manninotriose
Catalog No.:BCN8488
CAS No.:13382-86-0
- Dysolenticin J
Catalog No.:BCN7497
CAS No.:1337973-08-6
- GSK2656157
Catalog No.:BCC4983
CAS No.:1337532-29-2
- GSK2606414
Catalog No.:BCC1606
CAS No.:1337531-36-8
- Suavioside A
Catalog No.:BCN6963
CAS No.:133740-37-1
- Ebenifoline E-II
Catalog No.:BCN3097
CAS No.:133740-16-6
- GSK 2193874
Catalog No.:BCC8009
CAS No.:1336960-13-4
- 1-Allyl-3,5-Dimethylpyrazole
Catalog No.:BCC8450
CAS No.:13369-74-9
- Exoticin
Catalog No.:BCN6182
CAS No.:13364-94-8
- 2-Aminoisonicotinic acid
Catalog No.:BCC8550
CAS No.:13362-28-2
- 2-Ethyl-2,6,6-trimethylpiperidin-4-one
Catalog No.:BCN6504
CAS No.:133568-79-3
- Fmoc-Ile-ol
Catalog No.:BCC2584
CAS No.:133565-46-5
- SR 1664
Catalog No.:BCC6166
CAS No.:1338259-05-4
- Pseudolarolide Q2
Catalog No.:BCN8092
CAS No.:1338366-22-5
- EPZ004777
Catalog No.:BCC2218
CAS No.:1338466-77-5
- OTS514
Catalog No.:BCC4024
CAS No.:1338540-55-8
- OTS964
Catalog No.:BCC4025
CAS No.:1338545-07-5
- Safinamide
Catalog No.:BCC1915
CAS No.:133865-89-1
- Valnemulin HCl
Catalog No.:BCC4746
CAS No.:133868-46-9
- Soyasaponin Ac
Catalog No.:BCN2897
CAS No.:133882-74-3
- Eicosyl ferulate
Catalog No.:BCN4712
CAS No.:133882-79-8
- ML224
Catalog No.:BCC5596
CAS No.:1338824-21-7
- Kansuiphorin C
Catalog No.:BCN3764
CAS No.:133898-77-8
- Rimantadine
Catalog No.:BCC4938
CAS No.:13392-28-4
LIMK1/2 inhibitor LIMKi 3 suppresses porcine oocyte maturation.[Pubmed:27761340]
PeerJ. 2016 Oct 12;4:e2553.
LIMKi 3 is a specific selective LIMK inhibitor against LIMK1 and LIMK2, while LIMK1 and LIMK2 are the main regulators of actin cytoskeleton to participate in many cell activities. However, the effect of LIMKi 3 in porcine oocyte meiosis is still unclear. The present study was designed to investigate the effects of LIMKi 3 and potential regulatory role of LIMK1/2 on porcine oocyte meiotic maturation. Immunofluorescent staining of p-LIMK1/2 antibody showed that LIMK1/2 was localized mainly to the cortex of porcine oocyte, which co-localized with actin. After LIMKi 3 treatment, the diffusion of COCs became weak and the rate of polar body extrusion was decreased. This could be rescued by moving oocytes to fresh medium. After prolonging the culture time of oocytes, the maturation rate of porcine oocyte increased in LIMKi 3 groups, indicating that LIMKi 3 may suppress the cell cycle during porcine oocyte maturation. We also found that after LIMKi 3 treatment actin distribution was significantly disturbed at porcine oocyte membranes and cytoplasm, indicating the conserved roles of LIMK1/2 on actin dynamics. Next we examined the meiotic spindle positioning in porcine oocyte, and the results showed that a majority of spindles were not attached to the cortex of porcine oocyte, indicating that LIMKi 3 may affect actin-mediated spindle positioning. Taken together, these results showed that LIMK1/2 inhibitor LIMKi 3 had a repressive role on porcine oocyte meiotic maturation.
The actin-severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination.[Pubmed:22496574]
J Neurosci. 2012 Apr 11;32(15):5284-97.
Myelination is a complex process requiring coordination of directional motility and an increase in glial cell size to generate a multilamellar myelin sheath. Regulation of actin dynamics during myelination is poorly understood. However, it is known that myelin thickness is related to the abundance of neuregulin-1 (NRG1) expressed on the axon surface. Here we identify cofilin1, an actin depolymerizing and severing protein, as a downstream target of NRG1 signaling in rat Schwann cells (SCs). In isolated SCs, NRG1 promotes dephosphorylation of cofilin1 and its upstream regulators, LIM kinase (LIMK) and Slingshot-1 phosphatase (SSH1), leading to cofilin1 activation and recruitment to the leading edge of the plasma membrane. These changes are associated with rapid membrane expansion yielding a 35-50% increase in SC size within 30 min. Cofilin1-deficient SCs increase phosphorylation of ErbB2, ERK, focal adhesion kinase, and paxillin in response to NRG1, but fail to increase in size possibly due to stabilization of unusually long focal adhesions. Cofilin1-deficient SCs cocultured with sensory neurons do not myelinate. Ultrastructural analysis reveals that they unsuccessfully segregate or engage axons and form only patchy basal lamina. After 48 h of coculturing with neurons, cofilin1-deficient SCs do not align or elongate on axons and often form adhesions with the underlying substrate. This study identifies cofilin1 and its upstream regulators, LIMK and SSH1, as end targets of a NRG1 signaling pathway and demonstrates that cofilin1 is necessary for dynamic changes in the cytoskeleton needed for axon engagement and myelination by SCs.
LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells.[Pubmed:20876278]
J Cell Biol. 2010 Oct 4;191(1):169-85.
LIM kinases 1 and 2 (LIMK1/2) are centrally positioned regulators of actin cytoskeleton dynamics. Using siRNA-mediated knockdown or a novel small molecule inhibitor, we show LIMK is required for path generation by leading tumor cells and nontumor stromal cells during collective tumor cell invasion. LIMK inhibition lowers cofilin phosphorylation, F-actin levels, serum response factor transcriptional activity and collagen contraction, and reduces invasion in three-dimensional invasion assays. Although motility was unaffected, LIMK inhibition impairs matrix protein degradation and invadopodia formation associated with significantly faster recovery times in FRAP assays indicative of reduced F-actin stability. When LIMK is knocked down in MDA-MB-231 cells, they lose the ability to lead strands of collectively invading cells. Similarly, when LIMK activity is blocked in cancer-associated fibroblasts, they are unable to lead the collective invasion of squamous carcinoma cells in an organotypic skin model. These results show that LIMK is required for matrix remodeling activities for path generation by leading cells in collective invasion.
Identification of a nonkinase target mediating cytotoxicity of novel kinase inhibitors.[Pubmed:19001433]
Mol Cancer Ther. 2008 Nov;7(11):3490-8.
In developing inhibitors of the LIM kinases, the initial lead molecules combined potent target inhibition with potent cytotoxic activity. However, as subsequent compounds were evaluated, the cytotoxic activity separated from inhibition of LIM kinases. A rapid determination of the cytotoxic mechanism and its molecular target was enabled by integrating data from two robust core technologies. High-content assays and gene expression profiling both indicated an effect on microtubule stability. Although the cytotoxic compounds are still kinase inhibitors, and their structures did not predict tubulin as an obvious target, these results provided the impetus to test their effects on microtubule polymerization directly. Unexpectedly, we confirmed tubulin itself as a molecular target of the cytotoxic kinase inhibitor compounds. This general approach to mechanism of action questions could be extended to larger data sets of quantified phenotypic and gene expression data.


