GSK2656157PERK inhibitor CAS# 1337532-29-2 |
- PF-04691502
Catalog No.:BCC3837
CAS No.:1013101-36-4
- NVP-BGT226
Catalog No.:BCC3827
CAS No.:1245537-68-1
- HS-173
Catalog No.:BCC5363
CAS No.:1276110-06-5
- CUDC-907
Catalog No.:BCC2154
CAS No.:1339928-25-4
- PIK-93
Catalog No.:BCC2519
CAS No.:593960-11-3
- XL147
Catalog No.:BCC2487
CAS No.:956958-53-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

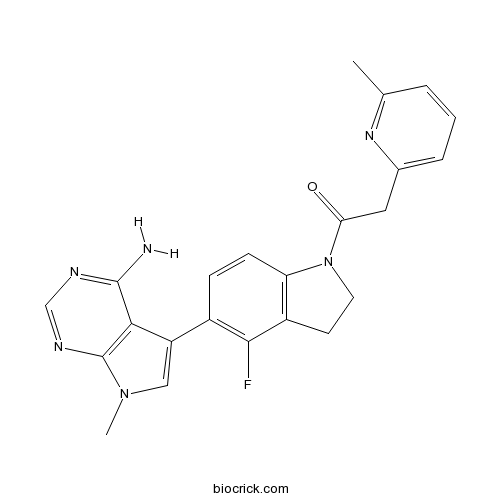
| Cas No. | 1337532-29-2 | SDF | Download SDF |
| PubChem ID | 53469059 | Appearance | Powder |
| Formula | C23H21FN6O | M.Wt | 416.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 41 mg/mL (98.45 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-[5-(4-amino-7-methylpyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoro-2,3-dihydroindol-1-yl]-2-(6-methylpyridin-2-yl)ethanone | ||
| SMILES | CC1=NC(=CC=C1)CC(=O)N2CCC3=C2C=CC(=C3F)C4=CN(C5=C4C(=NC=N5)N)C | ||
| Standard InChIKey | PRWSIEBRGXYXAJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H21FN6O/c1-13-4-3-5-14(28-13)10-19(31)30-9-8-16-18(30)7-6-15(21(16)24)17-11-29(2)23-20(17)22(25)26-12-27-23/h3-7,11-12H,8-10H2,1-2H3,(H2,25,26,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GSK2656157 is an ATP-competitive and highly selective inhibitor of PERK with an IC50 value of 0.9 nM. | |||||
| Targets | PERK | |||||
| IC50 | 0.9 nM | |||||

GSK2656157 Dilution Calculator

GSK2656157 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4012 mL | 12.0062 mL | 24.0125 mL | 48.025 mL | 60.0312 mL |
| 5 mM | 0.4802 mL | 2.4012 mL | 4.8025 mL | 9.605 mL | 12.0062 mL |
| 10 mM | 0.2401 mL | 1.2006 mL | 2.4012 mL | 4.8025 mL | 6.0031 mL |
| 50 mM | 0.048 mL | 0.2401 mL | 0.4802 mL | 0.9605 mL | 1.2006 mL |
| 100 mM | 0.024 mL | 0.1201 mL | 0.2401 mL | 0.4802 mL | 0.6003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK2656157 is a highly selective inhibitor of protein kinase R-like ER kinase (PERK) with IC50 value of 0.9nM [1].
GSK2656157 is highly selective for PERK enzyme against a panel of 300 kinases. In the BxPC3 pancreatic tumor cell line, treatment of GSK2656157 causes an inhibition of PERK and decreases in the downstream substrates, including phospho-eIF2α, ATF4 and CHOP. The inhibition of PERK results in effects on de novo protein synthesis as shown in BxPC3 cells [1].
In vivo assay shows that a single 50 mg/kg oral dose of GSK2656157 can completely inhibit the Thr980 phosphorylation of endogenous pancreatic PERK in mice. Furthermore, GSK2656157 causes dose-dependent inhibition of tumor growth in human tumor xenograft models of pancreatic cancer (BxPC3, HPAC and Capan2) and multiple myeloma (NCI-H929). Among these cancers, the Capan2 tumor is most sensitive [1].
References:
[1] Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, Choudhry AE, Alsaid H, Jucker BM, Axten JM, Kumar R. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013 Mar 15;73(6):1993-2002.
- GSK2606414
Catalog No.:BCC1606
CAS No.:1337531-36-8
- Suavioside A
Catalog No.:BCN6963
CAS No.:133740-37-1
- Ebenifoline E-II
Catalog No.:BCN3097
CAS No.:133740-16-6
- GSK 2193874
Catalog No.:BCC8009
CAS No.:1336960-13-4
- 1-Allyl-3,5-Dimethylpyrazole
Catalog No.:BCC8450
CAS No.:13369-74-9
- Exoticin
Catalog No.:BCN6182
CAS No.:13364-94-8
- 2-Aminoisonicotinic acid
Catalog No.:BCC8550
CAS No.:13362-28-2
- 2-Ethyl-2,6,6-trimethylpiperidin-4-one
Catalog No.:BCN6504
CAS No.:133568-79-3
- Fmoc-Ile-ol
Catalog No.:BCC2584
CAS No.:133565-46-5
- Boc-Threoninol(Bzl)
Catalog No.:BCC2704
CAS No.:133565-43-2
- Boc-Glutaminol
Catalog No.:BCC3092
CAS No.:133565-42-1
- Isoatriplicolide tiglate
Catalog No.:BCN7934
CAS No.:133559-39-4
- Dysolenticin J
Catalog No.:BCN7497
CAS No.:1337973-08-6
- Manninotriose
Catalog No.:BCN8488
CAS No.:13382-86-0
- LIMKi 3
Catalog No.:BCC7972
CAS No.:1338247-35-0
- SR 1664
Catalog No.:BCC6166
CAS No.:1338259-05-4
- Pseudolarolide Q2
Catalog No.:BCN8092
CAS No.:1338366-22-5
- EPZ004777
Catalog No.:BCC2218
CAS No.:1338466-77-5
- OTS514
Catalog No.:BCC4024
CAS No.:1338540-55-8
- OTS964
Catalog No.:BCC4025
CAS No.:1338545-07-5
- Safinamide
Catalog No.:BCC1915
CAS No.:133865-89-1
- Valnemulin HCl
Catalog No.:BCC4746
CAS No.:133868-46-9
- Soyasaponin Ac
Catalog No.:BCN2897
CAS No.:133882-74-3
- Eicosyl ferulate
Catalog No.:BCN4712
CAS No.:133882-79-8
Evidence for eIF2alpha phosphorylation-independent effects of GSK2656157, a novel catalytic inhibitor of PERK with clinical implications.[Pubmed:24401334]
Cell Cycle. 2014;13(5):801-6.
The endoplasmic reticulum (ER)-resident protein kinase PERK is a major component of the unfolded protein response (UPR), which promotes the adaptation of cells to various forms of stress. PERK phosphorylates the alpha subunit of the translation initiation factor eIF2 at serine 51, a modification that plays a key role in the regulation of mRNA translation in stressed cells. Several studies have demonstrated that the PERK-eIF2alpha phosphorylation pathway maintains insulin biosynthesis and glucose homeostasis, facilitates tumor formation and decreases the efficacy of tumor treatment with chemotherapeutic drugs. Recently, a selective catalytic PERK inhibitor termed GSK2656157 has been developed with anti-tumor properties in mice. Herein, we provide evidence that inhibition of PERK activity by GSK2656157 does not always correlate with inhibition of eIF2alpha phosphorylation. Also, GSK2656157 does not always mimic the biological effects of the genetic inactivation of PERK. Furthermore, cells treated with GSK2656157 increase eIF2alpha phosphorylation as a means to compensate for the loss of PERK. Using human tumor cells impaired in eIF2alpha phosphorylation, we demonstrate that GSK2656157 induces ER stress-mediated death suggesting that the drug acts independent of the inhibition of eIF2alpha phosphorylation. We conclude that GSK2656157 might be a useful compound to dissect pathways that compensate for the loss of PERK and/or identify PERK pathways that are independent of eIF2alpha phosphorylation.
GSK2656157, a PERK inhibitor, reduced LPS-induced IL-1beta production through inhibiting Caspase 1 activation in macrophage-like J774.1 cells.[Pubmed:27251848]
Immunopharmacol Immunotoxicol. 2016 Aug;38(4):298-302.
IL-1beta is one of the inflammatory cytokines and is cleaved from pro-IL-1beta proteolytically by activated Caspase 1. For the activation of Caspase 1, inflammasome was formed by two signals, what is called, priming and triggering signals. In this study, it was found that mouse macrophage J774.1 cells, when treated by single large amount of lipopolysaccharide (LPS), produced a significant amount of IL-1beta. On the other hand, IL-1beta production was not detected when treated by a single, small amount of LPS. Then, focusing on endoplasmic reticulum (ER) stress response among stress responses induced by a large amount of LPS, when GSK2656157, a PERK inhibitor, was used for inhibition of ER stress, GSK2656157 reduced IL-1beta production dose-dependently. Next, when Thapsigargin, an ER stress reagent, was added with LPS, IL-1beta production increased more than by LPS alone. Thus, these results suggested that ER stress was involved in LPS-induced IL-1beta production. When the activation of Caspase 1 was examined by fluorescence activated cell sorter analysis, it was found that GSK2656157 inhibited LPS-induced Caspase 1 activation. Further, it was confirmed that GSK2656157 did not affect LPS-induced TNF-alpha production and activation of NF-kappaB and specifically inhibited the PERK/eIF-2alpha pathway. Therefore, it was found that GSK2656157 specifically inhibited ER stress induced by large amount of LPS and reduced LPS-induced IL-1beta production through inhibition of Caspase 1 activation.
Discovery of GSK2656157: An Optimized PERK Inhibitor Selected for Preclinical Development.[Pubmed:24900593]
ACS Med Chem Lett. 2013 Aug 12;4(10):964-8.
We recently reported the discovery of GSK2606414 (1), a selective first in class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), which inhibited PERK activation in cells and demonstrated tumor growth inhibition in a human tumor xenograft in mice. In continuation of our drug discovery program, we applied a strategy to decrease inhibitor lipophilicity as a means to improve physical properties and pharmacokinetics. This report describes our medicinal chemistry optimization culminating in the discovery of the PERK inhibitor GSK2656157 (6), which was selected for advancement to preclinical development.


