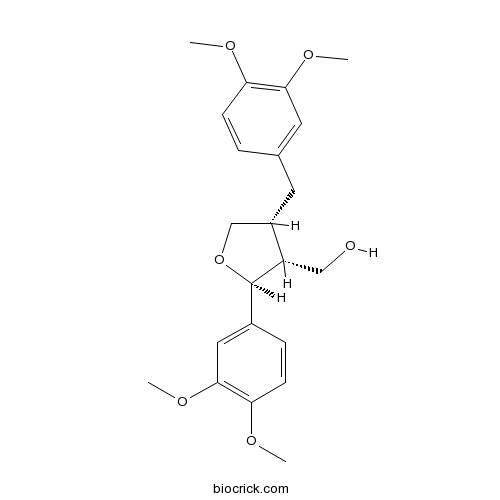
Lariciresinol dimethyl etherCAS# 67560-68-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 67560-68-3 | SDF | Download SDF |
| PubChem ID | 181325 | Appearance | Powder |
| Formula | C22H28O6 | M.Wt | 388.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2S,3R,4R)-2-(3,4-dimethoxyphenyl)-4-[(3,4-dimethoxyphenyl)methyl]oxolan-3-yl]methanol | ||
| SMILES | COC1=C(C=C(C=C1)CC2COC(C2CO)C3=CC(=C(C=C3)OC)OC)OC | ||
| Standard InChIKey | AYWPHVUFQNWITL-PNLZDCPESA-N | ||
| Standard InChI | InChI=1S/C22H28O6/c1-24-18-7-5-14(10-20(18)26-3)9-16-13-28-22(17(16)12-23)15-6-8-19(25-2)21(11-15)27-4/h5-8,10-11,16-17,22-23H,9,12-13H2,1-4H3/t16-,17-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | NO |

Lariciresinol dimethyl ether Dilution Calculator

Lariciresinol dimethyl ether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.574 mL | 12.87 mL | 25.74 mL | 51.4801 mL | 64.3501 mL |
| 5 mM | 0.5148 mL | 2.574 mL | 5.148 mL | 10.296 mL | 12.87 mL |
| 10 mM | 0.2574 mL | 1.287 mL | 2.574 mL | 5.148 mL | 6.435 mL |
| 50 mM | 0.0515 mL | 0.2574 mL | 0.5148 mL | 1.0296 mL | 1.287 mL |
| 100 mM | 0.0257 mL | 0.1287 mL | 0.2574 mL | 0.5148 mL | 0.6435 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PG 01037 dihydrochloride
Catalog No.:BCC7801
CAS No.:675599-62-9
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- Nutlin-3b
Catalog No.:BCC1156
CAS No.:675576-97-3
- 8'-Epicleomiscosin A
Catalog No.:BCC3917
CAS No.:
- Polygodial
Catalog No.:BCC7597
CAS No.:6754-20-7
- Dehydrotumulosic acid
Catalog No.:BCN3740
CAS No.:6754-16-1
- Helenalin
Catalog No.:BCN8073
CAS No.:6754-13-8
- Alpha-caryophyllene
Catalog No.:BCN3877
CAS No.:6753-98-6
- Thapsigargin
Catalog No.:BCC6952
CAS No.:67526-95-8
- 4-Methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile
Catalog No.:BCC8710
CAS No.:675126-26-8
- Spathulenol
Catalog No.:BCN4227
CAS No.:6750-60-3
- Arnidiol
Catalog No.:BCN3810
CAS No.:6750-30-7
- Maoyecrystal E
Catalog No.:BCN3283
CAS No.:675603-39-1
- Baicalein 7-O-beta-D-ethylglucuronide
Catalog No.:BCN7981
CAS No.:675624-38-1
- Diosbulbin G
Catalog No.:BCN4229
CAS No.:67567-15-1
- Hydrangenol 8-O-glucoside
Catalog No.:BCN4230
CAS No.:67600-94-6
- 5,7,2'-Trihydroxyflavanone
Catalog No.:BCC9241
CAS No.:120980-68-9
- Clerodermic acid methyl ester
Catalog No.:BCN4231
CAS No.:67650-47-9
- Methylenetanshinquinone
Catalog No.:BCN3156
CAS No.:67656-29-5
- 9-Hydroxycamptothecin
Catalog No.:BCC8276
CAS No.:67656-30-8
- 1-Azakenpaullone
Catalog No.:BCC5332
CAS No.:676596-65-9
- Syringylpropane
Catalog No.:BCN3540
CAS No.:6766-82-1
- De-O-methylacetovanillochromene
Catalog No.:BCN4232
CAS No.:67667-62-3
- 8alpha-(2-Methylacryloyloxy)hirsutinolide 13-O-acetate
Catalog No.:BCN7107
CAS No.:67667-71-4
Two new stereoisomers of Tetrahydrofuranoid lignans from the flower buds of Magnolia fargesii.[Pubmed:17202718]
Chem Pharm Bull (Tokyo). 2007 Jan;55(1):137-9.
Two new stereoisomers of tetrahydrofuranoid lignans, 7S,8R,7'S,8'R- (1) and 7R,8S,7'S,8'R-3,4,3',4'-tetramethoxy-9,7'-dihydroxy-8.8',7.O.9'-lignan (2) along with nine known lignans including tetrahydrofuranoids (3, 4) and tetrahydrofurofuranoids (5-11) were isolated from a CHCl(3)-soluble fraction of the flower buds of Magnolia fargesii. Two tetrahydrofuranoids, magnostellin A (3) and Lariciresinol dimethyl ether (4) were isolated from this species for the first time. The structures of these compounds (1-11) were identified by spectroscopic methods as well as by comparison with published values. Absolute configurations of new stereoisomers (1, 2) were determined by the Mosher's esterification method and Circular Dichroism (CD) studies. All the isolates (1-11) were evaluated for their antioxidant activities using modified superoxide radical-scavenging assay. Compounds 5-8 showed the potent superoxide radical-scavenging activities with the ED(50) values of 19.2, 19.2, 16.5, and 27.7 microM, respectively, as compared with standard antioxidants (BHA: 22.8 microM; Trolox: 940 microM).
Short and stereoselective total synthesis of furano lignans (+/-)-dihydrosesamin, (+/-)-lariciresinol dimethyl ether, (+/-)-acuminatin methyl ether, (+/-)-sanshodiol methyl ether, (+/-)-lariciresinol, (+/-)-acuminatin, and (+/-)-lariciresinol monomethyl ether and furofuran lignans (+/-)-sesamin, (+/-)-eudesmin, (+/-)-piperitol methyl ether, (+/-)-pinoresinol, (+/-)-piperitol, and (+/-)-pinoresinol monomethyl ether by radical cyclization of epoxides using a transition-metal radical source.[Pubmed:12003531]
J Org Chem. 2002 May 17;67(10):3242-8.
Intramolecular radical cyclization of suitably substituted epoxy ethers 4a-g using bis(cyclopentadienyl)titanium(III) chloride as the radical source resulted in trisubstituted tetrahydrofurano lignans and 2,6-diaryl-3,7-dioxabicyclo[3.3.0]octane lignans depending on the reaction conditions. The titanium(III) species was prepared in situ from commercially available titanocene dichloride and activated zinc dust in THF. Upon radical cyclization followed by acidic workup, epoxy olefinic ethers 4a-g afforded furano lignans dihydrosesamin 1a, Lariciresinol dimethyl ether 1b, acuminatin methyl ether 1e, and sanshodiol methyl ether 1g directly and lariciresinol 1h, acuminatin 1i, and lariciresinol monomethyl ether 1j after removal of the benzyl protecting group by controlled hydrogenolysis of the corresponding cyclized products. The furofuran lignans sesamin 2a, eudesmin 2b, and piperitol methyl ether 2e were also prepared directly by using the same precursors 4a-f on radical cyclization followed by treatment with iodine and pinoresinol 2h, piperitol 2i, and pinoresinol monomethyl ether 2j after controlled hydrogenolysis of the benzyl protecting group of the corresponding cyclized products. Two naturally occurring acyclic lignans, secoisolariciresinol 5h and secoisoLariciresinol dimethyl ether 5b, have also been prepared by exhaustive hydrogenolysis of 2h and 2b, respectively.


