Nutlin-3bMDM2/p53 inhibitor CAS# 675576-97-3 |
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- RG7112
Catalog No.:BCC1894
CAS No.:939981-39-2
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 675576-97-3 | SDF | Download SDF |
| PubChem ID | 16755649 | Appearance | Powder |
| Formula | C30H30Cl2N4O4 | M.Wt | 581.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Nutlin-3b | ||
| Solubility | DMSO : ≥ 100 mg/mL (171.97 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
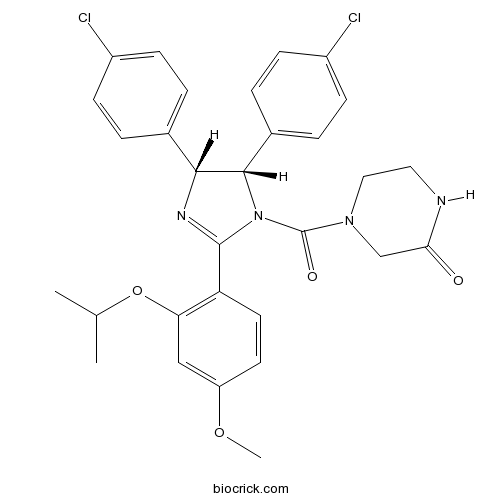
| Chemical Name | 4-[(4R,5S)-4,5-bis(4-chlorophenyl)-2-(4-methoxy-2-propan-2-yloxyphenyl)-4,5-dihydroimidazole-1-carbonyl]piperazin-2-one | ||
| SMILES | CC(C)OC1=C(C=CC(=C1)OC)C2=NC(C(N2C(=O)N3CCNC(=O)C3)C4=CC=C(C=C4)Cl)C5=CC=C(C=C5)Cl | ||
| Standard InChIKey | BDUHCSBCVGXTJM-IZLXSDGUSA-N | ||
| Standard InChI | InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nutlin-3b is an inhibitor of p53-MDM2 interaction with IC50 value of 13.6 µM. | |||||
| Targets | p53-MDM2 interaction | |||||
| IC50 | 13.6 µM | |||||

Nutlin-3b Dilution Calculator

Nutlin-3b Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7197 mL | 8.5986 mL | 17.1972 mL | 34.3944 mL | 42.993 mL |
| 5 mM | 0.3439 mL | 1.7197 mL | 3.4394 mL | 6.8789 mL | 8.5986 mL |
| 10 mM | 0.172 mL | 0.8599 mL | 1.7197 mL | 3.4394 mL | 4.2993 mL |
| 50 mM | 0.0344 mL | 0.172 mL | 0.3439 mL | 0.6879 mL | 0.8599 mL |
| 100 mM | 0.0172 mL | 0.086 mL | 0.172 mL | 0.3439 mL | 0.4299 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nutlin-3b is an inactive enantiomer of nutlin-3 [1].
Nutlin-3 is a small-molecule inhibitor of MDM2, interfering with MDM2-directedTP53 degradation. The stabilization of WT-TP53 leads to cell cycle arrest, growth inhibition, and apoptosis. As an inactive enantiomer, Nutlin-3b does not show any effect on the proliferation and has no insignificant effect on gene expression in cancer cells. Nutlin-3b binds MDM2 with about 200-fold lower affinity and is 150 times less active than 3a (an active enantiomer of nutlin-3). Nutlin-3b is usually used as a negative control. It shows no induction of MDM2, p53 or p21 expression and has no ability in colony-formation in H460 cell line. It also has no effect on cell cycle [1, 2].
References:
[1] Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1888-93.
[2] Cao C, Shinohara ET, Subhawong TK, Geng L, Kim KW, Albert JM, Hallahan DE, Lu B. Radiosensitization of lung cancer by nutlin, an inhibitor of murine double minute 2. Mol Cancer Ther. 2006 Feb;5(2):411-7.
- 8'-Epicleomiscosin A
Catalog No.:BCC3917
CAS No.:
- Polygodial
Catalog No.:BCC7597
CAS No.:6754-20-7
- Dehydrotumulosic acid
Catalog No.:BCN3740
CAS No.:6754-16-1
- Helenalin
Catalog No.:BCN8073
CAS No.:6754-13-8
- Alpha-caryophyllene
Catalog No.:BCN3877
CAS No.:6753-98-6
- Thapsigargin
Catalog No.:BCC6952
CAS No.:67526-95-8
- 4-Methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile
Catalog No.:BCC8710
CAS No.:675126-26-8
- Spathulenol
Catalog No.:BCN4227
CAS No.:6750-60-3
- Arnidiol
Catalog No.:BCN3810
CAS No.:6750-30-7
- Eupatolide
Catalog No.:BCN7345
CAS No.:6750-25-0
- Fuegin
Catalog No.:BCN5809
CAS No.:6750-10-3
- DIDS
Catalog No.:BCC7942
CAS No.:67483-13-0
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- PG 01037 dihydrochloride
Catalog No.:BCC7801
CAS No.:675599-62-9
- Lariciresinol dimethyl ether
Catalog No.:BCN4228
CAS No.:67560-68-3
- Maoyecrystal E
Catalog No.:BCN3283
CAS No.:675603-39-1
- Baicalein 7-O-beta-D-ethylglucuronide
Catalog No.:BCN7981
CAS No.:675624-38-1
- Diosbulbin G
Catalog No.:BCN4229
CAS No.:67567-15-1
- Hydrangenol 8-O-glucoside
Catalog No.:BCN4230
CAS No.:67600-94-6
- 5,7,2'-Trihydroxyflavanone
Catalog No.:BCC9241
CAS No.:120980-68-9
- Clerodermic acid methyl ester
Catalog No.:BCN4231
CAS No.:67650-47-9
- Methylenetanshinquinone
Catalog No.:BCN3156
CAS No.:67656-29-5
- 9-Hydroxycamptothecin
Catalog No.:BCC8276
CAS No.:67656-30-8
- 1-Azakenpaullone
Catalog No.:BCC5332
CAS No.:676596-65-9
d-Amino acid mutation of PMI as potent dual peptide inhibitors of p53-MDM2/MDMX interactions.[Pubmed:28916339]
Bioorg Med Chem Lett. 2017 Oct 15;27(20):4678-4681.
According to the previously reported potent dual l-peptide PMI of p53-MDM2/MDMX interactions, a series of d-amino acid mutational PMI analogues, PMI-1-4, with enhanced proteolytic resistence and in vitro tumor cell inhibitory activities were reported, of which Liposome-PMI-1 showed a stronger inhibitory activity against the U87 cell lines than Nutlin-3. This d-amino acid mutation strategy may give a hand for enhancing the potential of peptide drugs.
Sevoflurane Acts on Ubiquitination-Proteasome Pathway to Reduce Postsynaptic Density 95 Protein Levels in Young Mice.[Pubmed:28968276]
Anesthesiology. 2017 Dec;127(6):961-975.
BACKGROUND: Children with multiple exposures to anesthesia and surgery may have an increased risk of developing cognitive impairment. Sevoflurane, a commonly used anesthetic in children, has been reported to decrease levels of postsynaptic density 95 protein. However, the upstream mechanisms and downstream consequences of the sevoflurane-induced reduction in postsynaptic density 95 protein levels remains largely unknown. We therefore set out to assess whether sevoflurane acts on ubiquitination-proteasome pathway to facilitate postsynaptic density 95 protein degradation. METHODS: Six-day-old wild-type mice received anesthesia with 3% sevoflurane 2 h daily for 3 days starting on postnatal day 6. We determined the effects of the sevoflurane anesthesia on mRNA, protein and ubiquitinated levels of postsynaptic density 95 protein in neurons, and synaptosomes and hippocampus of young mice. Cognitive function in the mice was determined at postnatal day 31 by using a Morris water maze. Proteasome inhibitor MG132 and E3 ligase mouse double mutant 2 homolog inhibitor Nutlin-3 were used for the interaction studies. RESULTS: The sevoflurane anesthesia decreased protein, but not mRNA, levels of postsynaptic density 95, and reduced ubiquitinated postsynaptic density 95 protein levels in neurons, synaptosomes, and hippocampus of young mice. Both MG132 and Nutlin-3 blocked these sevoflurane-induced effects. Sevoflurane promoted the interaction of mouse double mutant 2 homolog and postsynaptic density 95 protein in neurons. Finally, MG132 and Nutlin-3 ameliorated the sevoflurane-induced cognitive impairment in the mice. CONCLUSIONS: These data suggest that sevoflurane acts on the ubiquitination-proteasome pathway to facilitate postsynaptic density 95 protein degradation, which then decreases postsynaptic density 95 protein levels, leading to cognitive impairment in young mice. These studies would further promote the mechanistic investigation of anesthesia neurotoxicity in the developing brain.
Nutlin-3 enhances the bortezomib sensitivity of p53-defective cancer cells by inducing paraptosis.[Pubmed:28798402]
Exp Mol Med. 2017 Aug 11;49(8):e365.
The proteasome inhibitor, bortezomib, is ineffective against many solid tumors. Nutlin-3 is a potent antagonist of human homolog of murine double minute 2/p53 interaction exhibiting promising therapeutic anti-cancer activity. In this study, we show that treatment of various p53-defective bortezomib-resistant solid tumor cells with bortezomib plus nutlin-3 induces paraptosis, which is a cell death mode accompanied by dilation of the endoplasmic reticulum (ER) and mitochondria. Bortezomib alone did not markedly alter cellular morphology, and nutlin-3 alone induced only a transient mitochondrial dilation. However, bortezomib/nutlin-3 co-treatment triggered the progressive fusion of swollen ER and the formation of megamitochondria, leading to cell death. Mechanistically, proteasomal-impairment-induced ER stress, CHOP upregulation and disruption of Ca(2+) homeostasis were found to be critically involved in the bortezomib/nutlin-3-induced dilation of the ER. Our results further suggest that mitochondrial unfolded protein stress may play an important role in the mitochondrial dilation observed during bortezomib/nutlin-3-induced cell death. Collectively, these findings suggest that bortezomib/nutlin-3 perturbs proteostasis, triggering ER/mitochondria stress and irrecoverable impairments in their structure and function, ultimately leading to paraptotic cell death.
Activation of the p53 Transcriptional Program Sensitizes Cancer Cells to Cdk7 Inhibitors.[Pubmed:29020632]
Cell Rep. 2017 Oct 10;21(2):467-481.
Cdk7, the CDK-activating kinase and transcription factor IIH component, is a target of inhibitors that kill cancer cells by exploiting tumor-specific transcriptional dependencies. However, whereas selective inhibition of analog-sensitive (AS) Cdk7 in colon cancer-derived cells arrests division and disrupts transcription, it does not by itself trigger apoptosis efficiently. Here, we show that p53 activation by 5-fluorouracil or nutlin-3 synergizes with a reversible Cdk7(as) inhibitor to induce cell death. Synthetic lethality was recapitulated with covalent inhibitors of wild-type Cdk7, THZ1, or the more selective YKL-1-116. The effects were allele specific; a CDK7(as) mutation conferred both sensitivity to bulky adenine analogs and resistance to covalent inhibitors. Non-transformed colon epithelial cells were resistant to these combinations, as were cancer-derived cells with p53-inactivating mutations. Apoptosis was dependent on death receptor DR5, a p53 transcriptional target whose expression was refractory to Cdk7 inhibition. Therefore, p53 activation induces transcriptional dependency to sensitize cancer cells to Cdk7 inhibition.
Synthesis and evaluation of modified chalcone based p53 stabilizing agents.[Pubmed:28743509]
Bioorg Med Chem Lett. 2017 Sep 1;27(17):4101-4106.
Tumor suppressor protein p53 induces cell cycle arrest and apoptotic cell death in response to various cellular stresses thereby preventing cancer development. Activation and stabilization of p53 through small organic molecules is, therefore, an attractive approach for the treatment of cancers retaining wild-type p53. In this context, a series of nineteen chalcones with various substitution patterns of functional groups including chloro, fluoro, methoxy, nitro, benzyloxy, 4-methyl benzyloxy was prepared using Claisen-Schmidt condensation. The compounds were characterized using NMR, HRMS, IR and melting points. Evaluation of synthesized compounds against human colorectal (HCT116) and breast (CAL-51) cancer cell lines revealed potent antiproliferative activities. Nine compounds displayed GI50 values in the low micromolar to submicromolar range; for example (E)-1-phenyl-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (SSE14108) showed GI50 of 0.473+/-0.043microM against HCT116 cells. Further analysis of these compounds revealed that (E)-3-(4-chlorophenyl)-1-phenylprop-2-en-1-one (SSE14105) and (E)-3-(4-methoxyphenyl)-1-phenylprop-2-en-1-one (SSE14106) caused rapid (4 and 8-h post-treatment) accumulation of p53 in HCT116 cells similar to its induction by positive control, Nutlin-3. Such activities were absent in 3-(4-methoxyphenyl)propiophenone (SSE14106H2) demonstrating the importance of conjugated ketone for antiproliferative and p53 stabilizing activity of the chalcones. We further evaluated p53 levels in the presence of cycloheximide (CHX) and the results showed that the p53 stabilization was regulated at post-translational level through blockage of its degradation. These chalcones can, therefore, act as fragment leads for further structure optimization to obtain more potent p53 stabilizing agents with enhanced anti-proliferative activities.


