DIDSanion transport inhibitor CAS# 67483-13-0 |
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 67483-13-0 | SDF | Download SDF |
| PubChem ID | 5702690 | Appearance | Powder |
| Formula | C16H8N2Na2O6S4 | M.Wt | 498.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO and to 10 mM in Potassium bicarbonate (0.1M) | ||
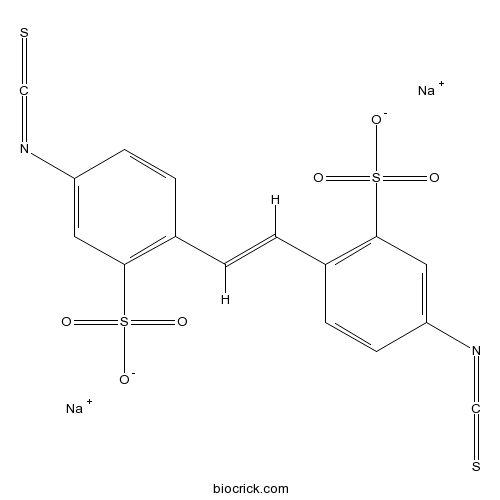
| Chemical Name | disodium;5-isothiocyanato-2-[(E)-2-(4-isothiocyanato-2-sulfonatophenyl)ethenyl]benzenesulfonate | ||
| SMILES | C1=CC(=C(C=C1N=C=S)S(=O)(=O)[O-])C=CC2=C(C=C(C=C2)N=C=S)S(=O)(=O)[O-].[Na+].[Na+] | ||
| Standard InChIKey | GEPAYBXVXXBSKP-SEPHDYHBSA-L | ||
| Standard InChI | InChI=1S/C16H10N2O6S4.2Na/c19-27(20,21)15-7-13(17-9-25)5-3-11(15)1-2-12-4-6-14(18-10-26)8-16(12)28(22,23)24;;/h1-8H,(H,19,20,21)(H,22,23,24);;/q;2*+1/p-2/b2-1+;; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ClC-Ka chloride channel blocker (IC50 = 100 μM). Blocks the maxi chloride channel in apical membranes from human placenta. Displays antagonistic activity at TRPM4 and TRPC4 channels; potentiates agonist-induced TRPV1 currents (IC50 = 4.88 μM in rat DRG neurons). Inhibits RAD51 recombinase activity (KD = 2 μM). |

DIDS Dilution Calculator

DIDS Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0061 mL | 10.0305 mL | 20.061 mL | 40.122 mL | 50.1525 mL |
| 5 mM | 0.4012 mL | 2.0061 mL | 4.0122 mL | 8.0244 mL | 10.0305 mL |
| 10 mM | 0.2006 mL | 1.003 mL | 2.0061 mL | 4.0122 mL | 5.0152 mL |
| 50 mM | 0.0401 mL | 0.2006 mL | 0.4012 mL | 0.8024 mL | 1.003 mL |
| 100 mM | 0.0201 mL | 0.1003 mL | 0.2006 mL | 0.4012 mL | 0.5015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
DIDS is an anion transport inhibitor, which inhibits the ClC-Ka chloride channel with an IC50 of 100 μM and the bacterial ClC-ec1Cl-/H+ exchanger with an IC50 of ~300 μM. [1]
Chloride channels are a superfamily consisting of approximately 13 subgroups and display a variety of functions in physiology. The human genome contains nine CLC proteins, which serve various physiological functions and potentially constitute novel exciting drug targets for the treatment of hypertension, osteoporosis, and gastrointestinal and renal disorders. [1]
DIDS’s effect on the calcium-activated chloride current [ICl (ca)] in muscle cells from the rabbit portal vein was studied with the perforated patch technique. Consequently, DIDS reduced the amplitude of spontaneous transient inward currents (STICs) in a concentration-dependent manner with an IC50 value of 2.1 x 10-4 M for STICs. Moreover, DIDS was investigated for its action on contraction of cerebral artery smooth muscle cells. DIDS showed a vasodilator effect on pressure-constricted arteries with IC50 of 69 ± 14 μM. [2, 3]
In vivo study showed DIDS alone increased the effect of hyperthermia at 42.5 ℃ or 43.5 ℃ to suppress tumor growth. The thermosensitization was greater when DIDS was combined with amiloride. Hyperthermia at 43.5 ℃ could result in a tumor growth delay for 4 days, while hyperthermia and treatment of 25 mg/kg DIDS prolonged the delay to approximately 6 days. As a proof, in vivo-in vitro excision assays for cell survival illustrated that DIDS enhanced the heat-induced tumor cell death. [4]
References:
[1] Wulff, Heike. "New light on the “Old” chloride channel blocker DIDS." ACS chemical biology 3.7 (2008): 399-401.
[2] Hogg, R. C., Q. Wang, and W. A. Large. "Effects of Cl channel blockers on Ca‐activated chloride and potassium currents in smooth muscle cells from rabbit portal vein." British journal of pharmacology 111.4 (1994): 1333-1341.
[3] Nelson, Mark T., et al. "Chloride channel blockers inhibit myogenic tone in rat cerebral arteries." The Journal of Physiology 502.2 (1997): 259-264.
[4] Lyons, John C., Brian D. Ross, and Chang W. Song. "Enhancement of hyperthermia effect in vivo by amiloride and DIDS." International Journal of Radiation Oncology* Biology* Physics 25.1 (1993): 95-103.
- GBR 12935 dihydrochloride
Catalog No.:BCC5380
CAS No.:67469-81-2
- Vanoxerine dihydrochloride
Catalog No.:BCC5129
CAS No.:67469-78-7
- GBR 12783 dihydrochloride
Catalog No.:BCC6676
CAS No.:67469-75-4
- Vanoxerine
Catalog No.:BCC5130
CAS No.:67469-69-6
- GBR 13069 dihydrochloride
Catalog No.:BCC6640
CAS No.:67469-45-8
- Amorolfine
Catalog No.:BCC8819
CAS No.:67467-83-8
- Isoasatone A
Catalog No.:BCN7762
CAS No.:67451-73-4
- Fmoc-Cys(tBu)-OH
Catalog No.:BCC3478
CAS No.:67436-13-9
- Isorhamnetin-3-O-galactoside
Catalog No.:BCC8190
CAS No.:6743-92-6
- (±)-Blebbistatin
Catalog No.:BCC7169
CAS No.:674289-55-5
- Z-D-His-OH
Catalog No.:BCC2767
CAS No.:67424-93-5
- 3-O-Acetyl-11-keto-beta-boswellic acid
Catalog No.:BCN1381
CAS No.:67416-61-9
- Fuegin
Catalog No.:BCN5809
CAS No.:6750-10-3
- Eupatolide
Catalog No.:BCN7345
CAS No.:6750-25-0
- Arnidiol
Catalog No.:BCN3810
CAS No.:6750-30-7
- Spathulenol
Catalog No.:BCN4227
CAS No.:6750-60-3
- 4-Methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile
Catalog No.:BCC8710
CAS No.:675126-26-8
- Thapsigargin
Catalog No.:BCC6952
CAS No.:67526-95-8
- Alpha-caryophyllene
Catalog No.:BCN3877
CAS No.:6753-98-6
- Helenalin
Catalog No.:BCN8073
CAS No.:6754-13-8
- Dehydrotumulosic acid
Catalog No.:BCN3740
CAS No.:6754-16-1
- Polygodial
Catalog No.:BCC7597
CAS No.:6754-20-7
- 8'-Epicleomiscosin A
Catalog No.:BCC3917
CAS No.:
- Nutlin-3b
Catalog No.:BCC1156
CAS No.:675576-97-3
A Joint Theoretical and Experimental Study of the Behavior of the DIDS Inhibitor and its Derivatives.[Pubmed:27062098]
Chemphyschem. 2016 Aug 4;17(15):2434-45.
4,4'-Diisothiocyanostilbene-2,2'-disulfonic acid (DIDS) is a well-known ion-exchange inhibitor targeting cardiac functions and indirectly impeding both radio- and chemo-resistance. A joint computational and experimental study is presented to provide deeper insights into DIDS and other members of this family of compounds. To this end, we applied state-of-the-art density functional theory (DFT) and time-dependent DFT methods, in addition to measuring the optical properties. The experimental data show that such compounds are highly sensitive to their environment and that the optical properties change within as little time as 7 h. However, the optical properties of DIDS are similar in various acidic/basic environments, which were confirmed by pKa computations on both cis and trans isomers. The protonation analysis also highlights that the singly protonated form of DIDS behaves like a proton sponge compound. The experimentally observed redshift that can be seen when going from water to DMSO was reproduced solely by using the solvation model based on density, although the polarization continuum model and implicit/explicit hybrid schemes were also tested. The characteristic broadening of the absorption peak in water and the vibronic fine structure in DMSO were also reproduced thanks to vibronic coupling simulations associated with the solvent reorganization energy. For other stilbene derivatives, a correlation is found between the maximum absorption wavelength and the Hammett parameters.
Drug-induced diseases (DIDs): An experience of a tertiary care teaching hospital from India.[Pubmed:26261164]
Indian J Med Res. 2015 Jul;142(1):33-9.
BACKGROUND & OBJECTIVES: Drug-induced diseases (DIDS) are well known but least studied. Data on DIDS from India are not available. Hence, this retrospective cross-sectional study was undertaken using suspected adverse drug reaction (ADR) data collected form Pharmacovigilance Programme of India (PvPI) to evaluate profile of DIDS over two years, in a tertiary care teaching hospital from north India. METHODS: The suspected ADRs in the form of DID were evaluated for drug and disease related variables and were classified in terms of causality. RESULTS: DID rate was 38.80 per cent. Mean duration of developing DIDS was 26.05 +/- 9.6 days; 25.16 per cent had more than one co-morbid condition. Geriatric population (53.99%) accounted for maximum DIDS followed by adult (37.79%) and paediatric (8.21%). Maximum events were probable (93.98%) followed by possible (6.04%). All DIDS required intervention. Gastritis (7.43%), diarrhoea (5.92%), anaemia (4.79%), hypotension (2.77%), hepatic dysfunction (2.69%), hypertension (1.51%), myalgia (1.05%), and renal dysfunction (1.01%) were some of the DIDS. Anti tubercular treatment (ATT), anti retroviral treatment (ART), ceftriaxone injection, steroids, non-steroidal anti-inflammatory drugs, antimicrobials and anticancer drugs were found as commonly offending drugs. INTERPRETATION & CONCLUSIONS: Our findings show that DIDS are a significant health problem in our country, which need more attention.
Al(3+) -promoted fluoride accumulation in tea plants (Camellia sinensis) was inhibited by an anion channel inhibitor DIDS.[Pubmed:26777729]
J Sci Food Agric. 2016 Sep;96(12):4224-30.
BACKGROUND: Generally, tea plants are grown in acid soil which is rich in aluminum (Al) and fluoride (F). A recent publication showed that pretreatment with Al(3+) promoted F accumulation in tea plants by increasing endogenous Ca(2+) and calmodulin (CaM). A high level of F in tea leaves not only impairs tea quality but also might pose a health risk for people drinking tea regularly. Therefore it is important to try to find some clues which might be beneficial in controlling F accumulation in tea plants grown in acid soil (Al(3+) ). RESULTS: It was found that diisothiocyanostilbene-2,2-disulfonic acid (DIDS) significantly reduced Al(3+) -promoted F accumulation in tea plants. Additionally, Al(3+) plus DIDS treatment stimulated significantly higher Ca(2+) efflux and decreased the CaM level in tea roots compared with Al(3+) treatment. Besides, significantly higher depolarization of membrane potential was shown in tea roots treated with Al(3+) plus DIDS than in those treated with Al(3+) , as well as higher net total H(+) efflux and plasma membrane H(+) -ATPase activity. CONCLUSION: Al(3+) -promoted F accumulation in tea plants was inhibited by an anion channel inhibitor DIDS. Ca(2+) /CaM and membrane potential depolarization may be the components involved in this process. (c) 2016 Society of Chemical Industry.
Agonist-dependent potentiation of vanilloid receptor transient receptor potential vanilloid type 1 function by stilbene derivatives.[Pubmed:22328719]
Mol Pharmacol. 2012 May;81(5):689-700.
Transient receptor potential vanilloid type 1 (TRPV1) is a nonselective cation channel activated by capsaicin, low pH, and noxious heat and plays a key role in nociception. Understanding mechanisms for functional modulation of TRPV1 has important implications. One characteristic of TRPV1 is that channel activity induced by either capsaicin or other activators can be sensitized or modulated by factors involving different cell signaling mechanisms. In this study, we describe a novel mechanism for the modulation of TRPV1 function: TRPV1 function is modulated by 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid (DIDS) and its analogs. We found that, in rat dorsal root ganglion neurons, although DIDS did not induce the activation of TRPV1 per se but drastically increased the TRPV1 currents induced by either capsaicin or low pH. DIDS also blocked the tachyphylaxis of the low pH-induced TRPV1 currents. 4-Acetamido-4'-isothiocyanatostilbene-2,2'-disulfonic acid (SITS), a DIDS analog, failed to enhance the capsaicin-evoked TRPV1 current but increased the low pH-evoked TRPV1 currents, with an effect comparable with that of DIDS. SITS also blocked the low pH-induced tachyphylaxis. DIDS also potentiated the currents of TRPV1 channels expressed in human embryonic kidney 293 cells, with an effect of left-shifting the concentration-response curve of the capsaicin-induced TRPV1 currents. This study demonstrates that DIDS and SITS, traditionally used chloride channel blockers, can modify TRPV1 channel function in an agonist-dependent manner. The results provide new input for understanding TRPV1 modulation and developing new modulators of TRPV1 function.
DIDS, a chemical compound that inhibits RAD51-mediated homologous pairing and strand exchange.[Pubmed:19336413]
Nucleic Acids Res. 2009 Jun;37(10):3367-76.
RAD51, an essential eukaryotic DNA recombinase, promotes homologous pairing and strand exchange during homologous recombination and the recombinational repair of double strand breaks. Mutations that up- or down-regulate RAD51 gene expression have been identified in several tumors, suggesting that inappropriate expression of the RAD51 activity may cause tumorigenesis. To identify chemical compounds that affect the RAD51 activity, in the present study, we performed the RAD51-mediated strand exchange assay in the presence of 185 chemical compounds. We found that 4,4'-diisothiocyanostilbene-2,2'-disulfonic acid (DIDS) efficiently inhibited the RAD51-mediated strand exchange. DIDS also inhibited the RAD51-mediated homologous pairing in the absence of RPA. A surface plasmon resonance analysis revealed that DIDS directly binds to RAD51. A gel mobility shift assay showed that DIDS significantly inhibited the DNA-binding activity of RAD51. Therefore, DIDS may bind near the DNA binding site(s) of RAD51 and compete with DNA for RAD51 binding.
New light on the "old" chloride channel blocker DIDS.[Pubmed:18642798]
ACS Chem Biol. 2008 Jul 18;3(7):399-401.
4,4'-Diisothiocyanatostilbene-2,2'-disulfonic acid (DIDS) has been used as an inhibitor of anion transporters and channels since the early 1970s. A study in this issue shows that DIDS hydrolyzes in aqueous solution and then multimerizes to di-, tri-, tetra-, and pentameric polythioureas, which inhibit both the bacterial ClC-ec1 Cl(-)/H(+) exchanger and the mammalian ClC-Ka chloride channel 3-200 times more potently than DIDS itself. The DIDS tetra- and pentamer could potentially act as tethered blockers that simultaneously obstruct both chloride pathways in the dimeric CLC proteins.
Regulation of human placental chloride channel by arachidonic acid and other cis unsaturated fatty acids.[Pubmed:9988821]
Am J Obstet Gynecol. 1999 Feb;180(2 Pt 1):469-75.
OBJECTIVE: Arachidonic acid has been implicated in the modulation of various transport processes, including conductive chloride transport in brush border membranes in the human placenta. The purpose of this work was to explore the effects of some cis unsaturated fatty acids on the electrophysiologic properties of the maxi chloride channels present in apical membranes from human placenta. STUDY DESIGN: Apical membrane chloride channels from human term placentas were reconstituted in giant liposomes. These cell-sized liposomes, generated by a cycle of dehydration and rehydration, are suitable for electrophysiologic studies by the patch-clamp method. RESULTS: Low micromolar concentrations of arachidonic acid reversibly inhibit maxi chloride channels in excised patches. Other cis unsaturated fatty acids, such as oleic and linoleic acids, show similar blockade. The inhibition was dose dependent. The maxi chloride channel can also be inhibited by 4,4 -diisothiocyanatostilbene-2,2 -disulfonic acid, a known chloride channel inhibitor. CONCLUSIONS: Our results identify the apical membrane maxi chloride channel as a possible electrophysi ologic counterpart of 4,4 -diisothiocyanatostilbene-2, 2 -disulfonic acid and cis unsaturated fatty acid-inhibited conductance previously described in brush border membranes of the human placenta. From a functional point of view the control of these channels by arachidonic acid may be of great importance in placental physiologic characteristics. Regulation of chloride channels could be important in the control of electrolyte and fluid transfer across the placenta. In addition, if these channels contribute to setting the membrane potential their regulation could have consequences for nutrient transport and delivery to the fetus. The electrophysiologic identification of these channels and their regulation might help to unravel their possible role in transplacental transport in normal and pathologic placental tissue.


