Loperamide HClCa2+ channel blocker (HVA) (L/N-type) CAS# 34552-83-5 |
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Tolcapone
Catalog No.:BCC2334
CAS No.:134308-13-7
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- FK866 (APO866)
Catalog No.:BCC2332
CAS No.:658084-64-1
- A922500
Catalog No.:BCC2333
CAS No.:959122-11-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34552-83-5 | SDF | Download SDF |
| PubChem ID | 71420 | Appearance | Powder |
| Formula | C29H34Cl2N2O2 | M.Wt | 513.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in ethanol and to 20 mM in DMSO | ||
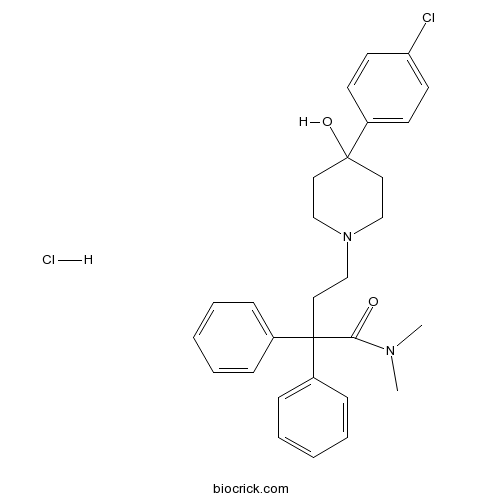
| Chemical Name | 4-(4-Chlorophenyl)-4-hydroxy-N,N-di | ||
| SMILES | [H+].[Cl-].CN(C)C(=O)C(CCN1CCC(O)(CC1)c2ccc(Cl)cc2)(c3ccccc3)c4ccccc4 | ||
| Standard InChIKey | PGYPOBZJRVSMDS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H33ClN2O2.ClH/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23;/h3-16,34H,17-22H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity μ-opioid receptor agonist with peripheral selectivity (Ki values are 2, 48 and 1156 nM for μ-, δ- and κ-opioid receptors respectively). Antidiarrhoeal and antihyperalgesic agent. Also a Ca2+ channel blocker; at low micromolar concentrations it blocks broad spectrum neuronal HVA Ca2+ channels and at higher concentrations it reduces Ca2+ flux through NMDA receptor operated channels. |

Loperamide HCl Dilution Calculator

Loperamide HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9474 mL | 9.7371 mL | 19.4742 mL | 38.9484 mL | 48.6855 mL |
| 5 mM | 0.3895 mL | 1.9474 mL | 3.8948 mL | 7.7897 mL | 9.7371 mL |
| 10 mM | 0.1947 mL | 0.9737 mL | 1.9474 mL | 3.8948 mL | 4.8685 mL |
| 50 mM | 0.0389 mL | 0.1947 mL | 0.3895 mL | 0.779 mL | 0.9737 mL |
| 100 mM | 0.0195 mL | 0.0974 mL | 0.1947 mL | 0.3895 mL | 0.4869 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Loperamide HCl is an opioid-receptor agonist used as long-acting synthetic antidiarrheals.
- Madecassoside
Catalog No.:BCN1012
CAS No.:34540-22-2
- 6,7-Dehydroferruginol
Catalog No.:BCN3218
CAS No.:34539-84-9
- Beta,beta-Dimethylacrylalkannin
Catalog No.:BCN2767
CAS No.:34539-65-6
- Arnicolide D
Catalog No.:BCN7975
CAS No.:34532-68-8
- Arnicolide C
Catalog No.:BCN7978
CAS No.:34532-67-7
- 1,3,6-Trihydroxy-2,5-dimethoxyxanthone
Catalog No.:BCN7216
CAS No.:345287-92-5
- Myricanol triacetate
Catalog No.:BCN5281
CAS No.:34509-52-9
- Pseudoephedrine Hydrochloride; Threo-Ephedrine Hydrochloride
Catalog No.:BCC8241
CAS No.:345-78-8
- SSR 69071
Catalog No.:BCC2369
CAS No.:344930-95-6
- Araloside X
Catalog No.:BCN2467
CAS No.:344911-90-6
- Leukadherin 1
Catalog No.:BCC6332
CAS No.:344897-95-6
- 17-ODYA
Catalog No.:BCC6717
CAS No.:34450-18-5
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- SF1670
Catalog No.:BCC5482
CAS No.:345630-40-2
- D-(+)-Mannose
Catalog No.:BCC8311
CAS No.:3458-28-4
- Ketotifen Fumarate
Catalog No.:BCC4531
CAS No.:34580-14-8
- Boc-Asp-OtBu
Catalog No.:BCC3073
CAS No.:34582-32-6
- SB 611812
Catalog No.:BCC6257
CAS No.:345892-71-9
- SUN-B 8155
Catalog No.:BCC7405
CAS No.:345893-91-6
- Amicarbalide
Catalog No.:BCC8117
CAS No.:3459-96-9
- MM 11253
Catalog No.:BCC7782
CAS No.:345952-44-5
- Wilfornine A
Catalog No.:BCN3099
CAS No.:345954-00-9
- AS601245
Catalog No.:BCC6464
CAS No.:345987-15-7
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
A multicenter double-blind controlled trial comparing lidamidine HCl and loperamide in the symptomatic treatment of acute diarrhoea.[Pubmed:3551969]
Arzneimittelforschung. 1986 Dec;36(12):1843-5.
The efficacy of 1-(2,6-dimethylphenyl)-3-methyl-amidinourea hydrochloride (lidamidine HCl, WHR-1142 A) an aryl-substituted amidinourea recently synthesized, was compared with that of loperamide in 32 patients with acute diarrhoea. The results of the study show that lidamidine HCl and loperamide had comparable effects in the pharmacological treatment of acute non-specific diarrhoea. Lidamidine HCl was also shown to be well tolerated; side-effects were generally minor and self-limiting.
Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity.[Pubmed:10087042]
J Pharmacol Exp Ther. 1999 Apr;289(1):494-502.
The antihyperalgesic properties of the opiate antidiarrheal agent loperamide (ADL 2-1294) were investigated in a variety of inflammatory pain models in rodents. Loperamide exhibited potent affinity and selectivity for the cloned micro (Ki = 3 nM) compared with the delta (Ki = 48 nM) and kappa (Ki = 1156 nM) human opioid receptors. Loperamide potently stimulated [35S]guanosine-5'-O-(3-thio)triphosphate binding (EC50 = 56 nM), and inhibited forskolin-stimulated cAMP accumulation (IC50 = 25 nM) in Chinese hamster ovary cells transfected with the human mu opioid receptor. The injection of 0.3 mg of loperamide into the intra-articular space of the inflamed rat knee joint resulted in potent antinociception to knee compression that was antagonized by naloxone, whereas injection into the contralateral knee joint or via the i.m. route failed to inhibit compression-induced changes in blood pressure. Loperamide potently inhibited late-phase formalin-induced flinching after intrapaw injection (A50 = 6 microgram) but was ineffective against early-phase flinching or after injection into the paw contralateral to the formalin-treated paw. Local injection of loperamide also produced antinociception against Freund's adjuvant- (ED50 = 21 microgram) or tape stripping- (ED50 = 71 microgram) induced hyperalgesia as demonstrated by increased paw pressure thresholds in the inflamed paw. In all animal models examined, the potency of loperamide after local administration was comparable to or better than that of morphine. Loperamide has potential therapeutic use as a peripherally selective opiate antihyperalgesic agent that lacks many of the side effects generally associated with administration of centrally acting opiates.
Maitotoxin-elicited calcium influx in cultured cells. Effect of calcium-channel blockers.[Pubmed:7488233]
Biochem Pharmacol. 1995 Oct 12;50(8):1187-97.
Maitotoxin elicited a marked influx of 45Ca2+ into NIH 3T3 fibroblast cells. The influx was blocked by imidazoles (econazole, miconazole, SKF 96365, clotrimazole, calmidazolium) with IC50 values from 0.56 to 3 microM. Phenylalkylamines (verapamil, methoxyverapamil) and nitrendipine were less potent, and diltiazem was very weak. Among other calcium blockers, the diphenylbutylpiperidines fluspirilene and penfluridol, the diphenylpropylpiperidine loperamide, and the local anesthetic proadifen were quite active with IC50 values of 2-4 microM. The pattern of inhibition of maitotoxin-elicited calcium influx did not correspond to the ability of the agents to block elevation of calcium that ensues through calcium-release activated calcium (CRAC) channels after activation of phosphoinositide breakdown by ATP in HL-60 cells. The imidazoles did block CRAC channels, but fluspirilene, penfluridol, loperamide and proadifen were ineffective. Loperamide actually appeared to enhance influx of calcium via the activated CRAC channels. The imidazoles, in particular calmidazolium, caused an apparent influx of calcium and caused a stimulation of phosphoinositide breakdown in HL-60 cells.
Loperamide blocks high-voltage-activated calcium channels and N-methyl-D-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal neurons.[Pubmed:8183255]
Mol Pharmacol. 1994 Apr;45(4):747-57.
The effects of the antidiarrheal agent loperamide on high-voltage-activated (HVA) calcium channel activity and excitatory amino acid-evoked responses in two preparations of cultured hippocampal pyramidal neurons were examined. In rat hippocampal neurons loaded with the calcium-sensitive dye fura-2, rises in intracellular free calcium concentration ([Ca2+]i) evoked by transient exposure to 50 mM K(+)-containing medium [high extracellular potassium concentration ([K+]o)] were mediated by Ca2+ flux largely through nifedipine-sensitive Ca2+ channels, with smaller contributions from omega-conotoxin GVIA (omega-CgTx)-sensitive Ca2+ channels and channels insensitive to both nifedipine and omega-CgTx. Loperamide reversibly blocked rises in [Ca2+]i evoked by high [K+]o in a concentration-dependent manner, with an IC50 of 0.9 +/- 0.2 microM. At the highest concentration tested (50 microM), loperamide eliminated rises in [Ca2+]i evoked by high [K+]o, a result otherwise achieved only in Ca(2+)-free medium or by the combined application of nifedipine, omega-CgTx, and funnel web spider venom to Ca(2+)-containing medium. The action of loperamide was neither naloxone sensitive nor mimicked by morphine and was seen at concentrations substantially less than those required to block influx of Ca2+ through the N-methyl-D-aspartate (NMDA) receptor-operated ionophore. Similar results were obtained in cultured mouse hippocampal pyramidal neurons under whole-cell voltage clamp. Voltage-activated Ca2+ channel currents carried by barium ions (IBa) could be discriminated pharmacologically into nifedipine-sensitive (L-type) and nifedipine-resistant, omega-CgTx-sensitive (N-type) components. Loperamide (0.1-50 microM) produced a concentration-dependent reduction of the peak IBa with an IC50 value of 2.5 +/- 0.4 microM and, at the highest concentration tested, could fully block IBa in the absence of any other pharmacological agent. The loperamide-induced block was rapid in onset and offset, was fully reversible, and did not appear to be related to the known calmodulin antagonist actions of loperamide. The current-voltage characteristics of the whole-cell IBa were unaffected by loperamide and the block was not voltage dependent. Loperamide also attenuated NMDA-evoked currents recorded at a membrane potential of -60 mV, with an IC50 of 73 +/- 7 microM. The block of NMDA-evoked currents was not competitive in nature, was not reversed by elevation of the extracellular glycine or spermine concentration, and was not affected by changes in the membrane holding potential. Steady state currents evoked by kainate and DL-alpha-amino-3-hydroxy-5-methylisoxazolepropionic acid were, in contrast, relatively unaffected by 100 microM loperamide.(ABSTRACT TRUNCATED AT 400 WORDS)


