Potassium CanrenoateAldosterone antagonist CAS# 2181-04-6 |
- Posaconazole hydrate
Catalog No.:BCC4234
CAS No.:1198769-38-8
Quality Control & MSDS
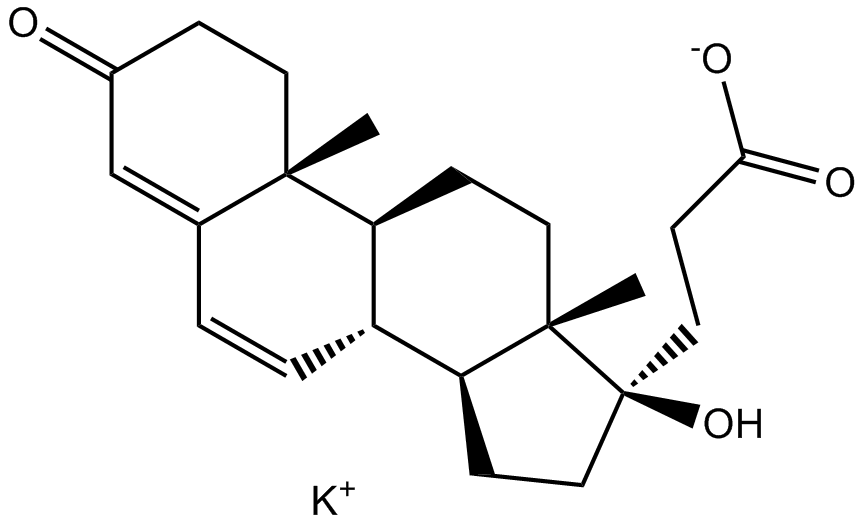
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2181-04-6 | SDF | Download SDF |
| PubChem ID | 23671691 | Appearance | Powder |
| Formula | C22H29KO4 | M.Wt | 396.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | <3.97mg/mL in DMSO | ||
| Chemical Name | potassium;3-[(8R,9S,10R,13S,14S,17R)-17-hydroxy-10,13-dimethyl-3-oxo-2,8,9,11,12,14,15,16-octahydro-1H-cyclopenta[a]phenanthren-17-yl]propanoate | ||
| SMILES | CC12CCC(=O)C=C1C=CC3C2CCC4(C3CCC4(CCC(=O)[O-])O)C.[K+] | ||
| Standard InChIKey | JTZQCHFUGHIPDF-RYVBEKKQSA-M | ||
| Standard InChI | InChI=1S/C22H30O4.K/c1-20-9-5-15(23)13-14(20)3-4-16-17(20)6-10-21(2)18(16)7-11-22(21,26)12-8-19(24)25;/h3-4,13,16-18,26H,5-12H2,1-2H3,(H,24,25);/q;+1/p-1/t16-,17+,18+,20+,21+,22-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Potassium Canrenoate Dilution Calculator

Potassium Canrenoate Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Potassium canrenoate is a competitive antagonist of aldosterone receptor [1].
Aldosterone is a steroid hormone and plays an important role in the regulation of blood pressure by increasing reabsorption of ions and water in the kidney.
In cultured rat and human cells, potassium canrenoate produced genotoxic effects in a doce-dependant way [2].
In male Wistar rats injected with isoprenaline (400 mg/kg), heart and left ventricular weights increased significantly. While potassium canrenoate (20 mg/kg/day) relieved this increase. Potassium canrenoate significantly reduced the fibrosis induced by isoprenaline [1]. In 30 essential hypertensives, canrenoate potassium reduced intraerythrocyte Na+ content and increased Na-K pump [3]. In 15 patients with idiopathic primary aldosteronism, potassium canrenoate normalized the aldosterone/plasma renin activity (PRA) ratio [4].
References:
[1]. Bos R, Mougenot N, Médiani O, et al. Potassium canrenoate, an aldosterone receptor antagonist, reduces isoprenaline-induced cardiac fibrosis in the rat. J Pharmacol Exp Ther, 2004, 309(3): 1160-1166.
[2]. Martelli A, Mattioli F, Carrozzino R, et al. Genotoxicity testing of potassium canrenoate in cultured rat and human cells. Mutagenesis, 1999, 14(5): 463-472.
[3]. Niutta E, Cusi D, Colombo R, et al. Antihypertensive effect of captopril, canrenoate potassium, and atenolol. Relations with red blood cell sodium transport and renin. Am J Hypertens, 1988, 1(4 Pt 1): 364-371.
[4]. Armanini D, Scaroni C, Mattarello MJ, et al. Idiopathic primary hyperaldosteronism: normalization of plasma aldosterone after one month withdrawal of long-term therapy with aldosterone-receptor antagonist potassium canrenoate. J Endocrinol Invest, 2005, 28(3): 236-240.
- Thalifoline
Catalog No.:BCN3301
CAS No.:21796-15-6
- Noroxyhydrastinine
Catalog No.:BCN2646
CAS No.:21796-14-5
- Homomangiferin
Catalog No.:BCN8145
CAS No.:21794-66-1
- Diallyl disulfide
Catalog No.:BCN3840
CAS No.:2179-57-9
- Curcolonol
Catalog No.:BCN3558
CAS No.:217817-09-9
- 9,16-Dioxo-10,12,14-octadecatrienoic acid
Catalog No.:BCN1490
CAS No.:217810-46-3
- H-Asp(OBzl)-OH
Catalog No.:BCC2885
CAS No.:2177-63-1
- 4-Cadinen-7-ol
Catalog No.:BCN4932
CAS No.:217650-27-6
- BX 471
Catalog No.:BCC6029
CAS No.:217645-70-0
- Taxoquinone
Catalog No.:BCN6660
CAS No.:21764-41-0
- Cyclo(Ala-Tyr)
Catalog No.:BCN2412
CAS No.:21754-26-7
- Cyclo(Tyr-Val)
Catalog No.:BCN2413
CAS No.:21754-25-6
- Isopteleine
Catalog No.:BCN7067
CAS No.:2181-84-2
- SC 26196
Catalog No.:BCC7880
CAS No.:218136-59-5
- SRPIN340
Catalog No.:BCC4849
CAS No.:218156-96-8
- Vindoline
Catalog No.:BCN4933
CAS No.:2182-14-1
- Nifedipine
Catalog No.:BCC4808
CAS No.:21829-25-4
- Macrocarpal K
Catalog No.:BCN4934
CAS No.:218290-59-6
- BDNF (human)
Catalog No.:BCC5944
CAS No.:218441-99-7
- Octahydroisoindole
Catalog No.:BCN2275
CAS No.:21850-12-4
- Falcarinol
Catalog No.:BCN3938
CAS No.:21852-80-2
- Bardoxolone
Catalog No.:BCC1399
CAS No.:218600-44-3
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Boc- ß-HoIle-OH
Catalog No.:BCC3236
CAS No.:218608-82-3
Potassium canrenoate treatment in paediatric patients: a population pharmacokinetic study using novel dried blood spot sampling.[Pubmed:23846862]
J Hypertens. 2013 Sep;31(9):1901-8.
OBJECTIVE: To characterize the population pharmacokinetics of canrenone following administration of Potassium Canrenoate (K-canrenoate) in paediatric patients. METHODS: Data were collected prospectively from 37 paediatric patients (median weight 2.9 kg, age range 2 days-0.85 years) who received intravenous K-canrenoate for management of retained fluids, for example in heart failure and chronic lung disease. Dried blood spot (DBS) samples (n=213) from these were analysed for canrenone content and the data subjected to pharmacokinetic analysis using nonlinear mixed-effects modelling. Another group of patients (n=16) who had 71 matching plasma and DBS samples was analysed separately to compare canrenone pharmacokinetic parameters obtained using the two different matrices. RESULTS: A one-compartment model best described the DBS data. Significant covariates were weight, postmenstrual age (PMA) and gestational age. The final population models for canrenone clearance (CL/F) and volume of distribution (V/F) in DBS were CL/F (l/h) = 12.86 x (WT/70.0) x e [0.066 x (PMA - 40]) and V/F (l) = 603.30 x (WT/70) x (GA/40) where weight is in kilograms. The corresponding values of CL/F and V/F in a patient with a median weight of 2.9 kg are 1.11 l/h and 20.48 l, respectively. Estimated half-life of canrenone based on DBS concentrations was similar to that based on matched plasma concentrations (19.99 and 19.37 h, respectively, in 70 kg patient). CONCLUSION: The range of estimated CL/F in DBS for the study population was 0.12-9.62 l/h; hence, bodyweight-based dosage adjustment of K-canrenoate appears necessary. However, a dosing scheme that takes into consideration both weight and age (PMA/gestational age) of paediatric patients seems more appropriate.
Electrophysiological and antiarrhythmic properties of potassium canrenoate during myocardial ischemia-reperfusion.[Pubmed:25389106]
J Cardiovasc Pharmacol Ther. 2015 May;20(3):313-21.
INTRODUCTION: Recent clinical studies have reported the potential benefit of an early mineralocorticoid receptor (MR) blockade with Potassium Canrenoate (PC) on ventricular arrhythmias (VAs) occurrence in patients experiencing an ST-segment elevation myocardial infarction (STEMI). However, most of the electrophysiological properties of PC demonstrated to date have been investigated in normoxic conditions, and therefore, in vitro experiments during an acute myocardial ischemia-reperfusion were lacking. MATERIALS AND METHODS: We used rabbit in vitro models and standard microelectrode technique to assess the electrophysiological impact of PC during myocardial ischemia-reperfusion, including right ventricle mimicking the "border zone" existing between normal and ischemic/reperfused areas (1 micromol/L, 10 and 100 nmol/L), isolated right ventricle, and sinoatrial node (SAN) experiments (1 micromol/L, respectively). RESULTS: During ischemia-reperfusion, acute superfusion of PC 100 nmol/L prevented the increase in action potential (AP) duration at 90% of repolarization (APD90) dispersion between ischemic and nonischemic areas and in VAs occurrence induced by aldosterone 10 nmol/L (86 +/- 3 vs 114 +/- 4 milliseconds for aldosterone alone, P < .05). Potassium Canrenoate also induced conduction blocks and significantly decreased Vmax during simulated ischemia (from 25 +/- 5 to 12 +/- 4, 14 +/- 3, and 14 +/- 5 V/s, respectively, for PC 1 micromol/L, 100, and 10 nmol/L, P < .05). Potassium Canrenoate 1 micromol/L demonstrated cycle length (CL)-dependent effects on APD90 and on Vmax, and it also reduced SAN beating CL (from 446 +/- 28 to 529 +/- 24 millisecond, P < .05). CONCLUSION: Our experimental study highlights new evidence for an antiarrhythmic impact of PC during myocardial ischemia-reperfusion via multiple channels modulation. These results are in line with recent clinical trials suggesting that an early MR blockade in STEMI may be preventive of VAs.
Population pharmacokinetic model of canrenone after intravenous administration of potassium canrenoate to paediatric patients.[Pubmed:22376078]
Br J Clin Pharmacol. 2012 Nov;74(5):864-72.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT: Little is known about the pharmacokinetics of Potassium Canrenoate/canrenone in paediatric patients WHAT THIS STUDY ADDS: A population pharmacokinetic model has been developed to evaluate the pharmacokinetics of canrenone in paediatric patients who received Potassium Canrenoate as part of their therapy in the intensive care unit. AIMS To characterize the population pharmacokinetics of canrenone following administration of Potassium Canrenoate to paediatric patients. METHODS: Data were collected prospectively from 23 paediatric patients (2 days to 10 years of age; median weight 4 kg, range 2.16-28.0 kg) who received intravenous Potassium Canrenoate (K-canrenoate) as part of their intensive care therapy for removal of retained fluids, e.g. in pulmonary oedema due to chronic lung disease and for the management of congestive heart failure. Plasma samples were analyzed by HPLC for determination of canrenone (the major metabolite and pharmacologically active moiety) and the data subjected to pharmacokinetic analysis using NONMEM. RESULTS: A one compartment model best described the data. The only significant covariate was weight (WT). The final population models for canrenone clearance (CL/F) and volume of distribution (V/F) were CL/F (l h(-1) ) = 11.4 x (WT/70.0)(0.75) and V/F (l) = 374.2 x (WT/70) where WT is in kg. The values of CL/F and V/F in a 4 kg child would be 1.33 l h(-1) and 21.4 l, respectively, resulting in an elimination half-life of 11.2 h. CONCLUSIONS: The range of estimated CL/F in the study population was 0.67-7.38 l h(-1) . The data suggest that adjustment of K-canrenoate dosage according to body weight is appropriate in paediatric patients.
Clinically insignificant negative interferences of spironolactone, potassium canrenoate, and their common metabolite canrenone in new dimension vista LOCI digoxin immunoassay.[Pubmed:22628228]
J Clin Lab Anal. 2012 May;26(3):143-7.
Spironolactone, a potassium-sparing diuretic metabolized to canrenone is often used with digoxin to treat various conditions including congestive heart failure. Potassium Canrenoate is a similar drug, which is also metabolized to canrenone. Due to reported both positive and negative interference of spironolactone, Potassium Canrenoate, and their common metabolite canrenone with digoxin immunoassays, we investigated potential interference of these compounds with the new homogenous sequential chemiluminescent assay for digoxin based on the luminescent oxygen channeling technology (LOCI digoxin) for application on the Dimension and Vista platform. When aliquots of a drug-free serum pool were supplemented with various amounts of spironolactone, Potassium Canrenoate, or canrenone and apparent digoxin values were measured using Dimension Vista LOCI digoxin assay, we observed no detected value except when aliquots were supplemented with very high amounts of Potassium Canrenoate or canrenone. However, we observed that apparent digoxin concentrations were very low. When aliquots of a serum digoxin pool (prepared by pooling specimens from patients receiving digoxin), were further supplemented with various amounts of spironolactone, Potassium Canrenoate, or canrenone and serum digoxin concentrations were remeasured using the LOCIdigoxin assay, only statistically significant falsely lower digoxin values (negative interference) were observed in specimens containing very high amounts of canrenone or Potassium Canrenoate. However, such small bias may not have any clinical significance. We conclude that new Dimension Vista LOCI digoxin assay is virtually free from interferences of spironolactone, Potassium Canrenoate, and their common metabolite canrenone.


