RO4929097γ secretase inhibitor CAS# 847925-91-1 |
- gamma-secretase modulator 2
Catalog No.:BCC1584
CAS No.:1093978-89-2
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

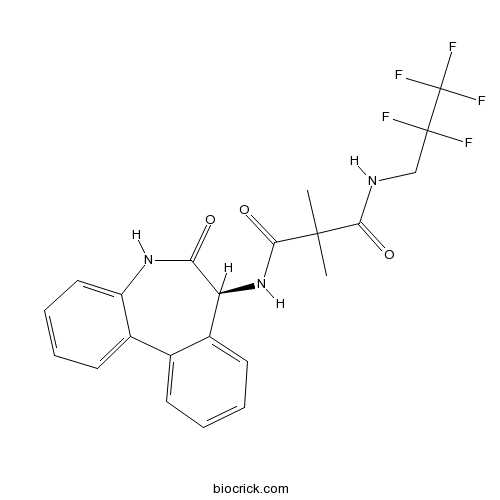
| Cas No. | 847925-91-1 | SDF | Download SDF |
| PubChem ID | 49867930 | Appearance | Powder |
| Formula | C22H20F5N3O3 | M.Wt | 469.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 49 mg/mL (104.39 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2,2-dimethyl-N-[(7S)-6-oxo-5,7-dihydrobenzo[d][1]benzazepin-7-yl]-N'-(2,2,3,3,3-pentafluoropropyl)propanediamide | ||
| SMILES | CC(C)(C(=O)NCC(C(F)(F)F)(F)F)C(=O)NC1C2=CC=CC=C2C3=CC=CC=C3NC1=O | ||
| Standard InChIKey | OJPLJFIFUQPSJR-INIZCTEOSA-N | ||
| Standard InChI | InChI=1S/C22H20F5N3O3/c1-20(2,18(32)28-11-21(23,24)22(25,26)27)19(33)30-16-14-9-4-3-7-12(14)13-8-5-6-10-15(13)29-17(16)31/h3-10,16H,11H2,1-2H3,(H,28,32)(H,29,31)(H,30,33)/t16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | RO4929097 is an inhibitor of γ secretase with IC50 of 4 nM, inhibiting cellular processing of Aβ40 and Notch with EC50 of 14 nM and 5 nM, respectively. | |||||
| Targets | γ secretase | Aβ40 | ICN | |||
| IC50 | 4 nM | 14 nM | 5 nM | |||
| Cell experiment: [1] | |
| Cell lines | SUM190 and SUM149 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 1 μM, 14 days for 2D cultures 7 days for 3D cultures |
| Applications | After treating with increasing doses of ionizing radiation in the presence or absence of the drug, 2D colonies were allowed to grow for 10–14 days, while the mammospheres were permitted to grow for 1 week. At 1 μM, RO4929097 was able to sensitize adherent cells to radiation with a more significant effect seen in SUM190 than in SUM149 cells. However, the same dose of inhibitor radioprotected cells grown under conditions that favor the enrichment of the cancer stem cells at higher doses of ionizing radiation. This discrepancy between 2D and 3D cultures suggested that cell contact may be needed for a Notch inhibitor to have a significant effect. |
| Animal experiment: [2] | |
| Animal models | NOD/SCID/IL2gammaR-/- (NOG) mice injected with WM3248 cells |
| Dosage form | Oral administration, 10 mg/Kg/day for 30 days |
| Application | There was a decrease in tumor growth with RO4929097 treatment, which was more appreciable after tumors were extracted for weight assessment. RO4929097-treated tumors also displayed lower expression of putative melanoma stem cell markers CD166, CD271 and JARID1B compared to vehicle-treated ones. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Debeb B G, Cohen E N, Boley K, et al. Pre-clinical studies of Notch signaling inhibitor RO4929097 in inflammatory breast cancer cells. Breast cancer research and treatment, 2012, 134(2): 495-510. [2] Huynh C, Poliseno L, Segura M F, et al. The novel gamma secretase inhibitor RO4929097 reduces the tumor initiating potential of melanoma. PloS one, 2011, 6(9): e25264. | |

RO4929097 Dilution Calculator

RO4929097 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1304 mL | 10.6519 mL | 21.3038 mL | 42.6076 mL | 53.2595 mL |
| 5 mM | 0.4261 mL | 2.1304 mL | 4.2608 mL | 8.5215 mL | 10.6519 mL |
| 10 mM | 0.213 mL | 1.0652 mL | 2.1304 mL | 4.2608 mL | 5.3259 mL |
| 50 mM | 0.0426 mL | 0.213 mL | 0.4261 mL | 0.8522 mL | 1.0652 mL |
| 100 mM | 0.0213 mL | 0.1065 mL | 0.213 mL | 0.4261 mL | 0.5326 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The development of the combination therapy of RO4949097 and cediranib is based on the understanding of the Notch signaling’s role in tumorigenesis and drug resistance.
Abstract
The recommended dose, safety, PKs and pharmacodynamics of RO4929097 plus temsirolimus are to be explored.
Abstract
The RO4929097 binding in plasma and its potential impact on the pharmacokinetics and pharmacodynamics of RO4929097 were investigated.
Abstract
The anti-melanoma activity of RO4929097, a GSI catalyzing the cleavage of the Notch receptor, is associated with PTEN expression, where RO4929097 alone or in combination with chemotherapy induces apoptosis through reducing AKT phosphorylation in melanoma cells expressing PTEN.
Abstract
RO4929097, a γ-secretase inhibitor was assessed for its activity in patients with metastatic, refractory colorectal cancer.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
RO4929097 is a small-molecule inhibitor of γ secretase with IC50 of 4 nM and EC50 of 5 nM [1]. It shows no in vitro inhibitory activity on the closely related proteases. It also has greater than 100-fold selectivity with respect to 75 other proteins of various types [1]. RO4929097 binds to γ secretase and inhibits its protease activity, therefore blocking the cleavage of Notch and reducing Notch signaling. Up-regulaton of this signaling pathway promotes tumorigenesis of multiple cancers.
RO4929097 has shown potential antitumor activity both in vitro and in vivo. It impaired the growth of melanoma cell lines and tumor formation of human primary melanoma xenograft [2]. It slowed proliferation and reduces colony formation of breast cancer cell lines[1]. In addition, RO4929097 decreased tumor formation in xenograft models of colorectal, pancreatic, lung cancer and melanoma[1, 2]. RO4929097 has been tested in multiple phase I/II clinical trials in patients with advanced solid tumors, either as monotherapy or in combination with other anti-tumor agents[3-8].
References:
1. Luistro L, He W, Smith M et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res 2009; 69: 7672-7680.
2. Huynh C, Poliseno L, Segura MF et al. The novel gamma secretase inhibitor RO4929097 reduces the tumor initiating potential of melanoma. PLoS One 2011; 6: e25264.
3. Richter S, Bedard PL, Chen EX et al. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Invest New Drugs 2014; 32: 243-249.
4. Sahebjam S, Bedard PL, Castonguay V et al. A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503). Br J Cancer 2013; 109: 943-949.
5. Diaz-Padilla I, Hirte H, Oza AM et al. A phase Ib combination study of RO4929097, a gamma-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest New Drugs 2013; 31: 1182-1191.
6. Tolcher AW, Messersmith WA, Mikulski SM et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 2012; 30: 2348-2353.
7. Strosberg JR, Yeatman T, Weber J et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer 2012; 48: 997-1003.
8. Kolb EA, Gorlick R, Keir ST et al. Initial testing (stage 1) by the pediatric preclinical testing program of RO4929097, a gamma-secretase inhibitor targeting notch signaling. Pediatr Blood Cancer 2012; 58: 815-818.
- Lenalidomide hemihydrate
Catalog No.:BCC4198
CAS No.:847871-99-2
- S 32212 hydrochloride
Catalog No.:BCC6208
CAS No.:847871-78-7
- Tasumatrol L
Catalog No.:BCN6955
CAS No.:847835-17-0
- ICG 001
Catalog No.:BCC3632
CAS No.:847591-62-2
- NVP-BEP800
Catalog No.:BCC2129
CAS No.:847559-80-2
- SHA 68
Catalog No.:BCC6210
CAS No.:847553-89-3
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Eriocalyxin B
Catalog No.:BCN4390
CAS No.:84745-95-9
- Qingyangshengenin
Catalog No.:BCN4389
CAS No.:84745-94-8
- Calceolarioside A
Catalog No.:BCN5347
CAS No.:84744-28-5
- UVI 3003
Catalog No.:BCC7638
CAS No.:847239-17-2
- Arglabin
Catalog No.:BCC5299
CAS No.:84692-91-1
- Lck Inhibitor
Catalog No.:BCC1689
CAS No.:847950-09-8
- 5-Epilithospermoside
Catalog No.:BCN4392
CAS No.:84799-31-5
- threo-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1331
CAS No.:848031-94-7
- 3,4-Dimethoxyphenyl beta-D-glucoside
Catalog No.:BCN4393
CAS No.:84812-00-0
- HKI 357
Catalog No.:BCC6046
CAS No.:848133-17-5
- Alvelestat
Catalog No.:BCC4058
CAS No.:848141-11-7
- EX-527 S-enantiomer
Catalog No.:BCC5594
CAS No.:848193-68-0
- EX-527 R-enantiomer
Catalog No.:BCC5595
CAS No.:848193-69-1
- SSR128129E
Catalog No.:BCC4498
CAS No.:848318-25-2
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- NCH 51
Catalog No.:BCC2422
CAS No.:848354-66-5
- Vasicinolone
Catalog No.:BCN4394
CAS No.:84847-50-7
Inhibition of the NOTCH pathway using gamma-secretase inhibitor RO4929097 has limited antitumor activity in established glial tumors.[Pubmed:25486598]
Anticancer Drugs. 2015 Mar;26(3):272-83.
Notch signaling is altered in many cancers. Our previous findings in primary pediatric ependymoma support a role for NOTCH in glial oncogenesis. The present study evaluates the gamma-secretase inhibitor RO4929097 in glial tumor models. The expression of Notch pathway genes was evaluated using real-time RT-PCR in 21 ependymoma and glioma models. NOTCH1 mutations were analyzed by DNA sequencing. RO4929097 activity was evaluated in vitro and in vivo, as a single agent and in combination, in glioma and ependymoma models. Notch pathway genes are overexpressed in ependymomas and gliomas along with FBXW7 downregulation. NOTCH1 mutations in the TAD domain were observed in 20% (2/10) of ependymoma primary cultures. Blocking the Notch pathway with the gamma-secretase inhibitor RO4929097 reduced cell density and viability in ependymoma short-term cultures. When combined with chemotherapeutic agents, RO4929097 enhanced temozolomide effects in ependymoma short-term cultures and potentiated the cytotoxicity of etoposide, cisplatinum, and temozolomide in glioma cells. RO4929097, in combined treatment with mTOR inhibition, potentiated cytotoxicity in vitro, but did not enhance antitumor effects in vivo. In contrast, RO4929097 enhanced irradiation effects in glioma and ependymoma xenografts and showed tumor growth inhibition in advanced-stage IGRG121 glioblastoma xenografts. RO4929097-mediated effects were independent of NOTCH1 mutation status or expression levels, but associated with low IL-6 levels. In established glial tumor models, NOTCH inhibition had limited effects as a single agent, but enhanced efficacy when combined with DNA-interfering agents. These preclinical data need to be considered for further clinical development of NOTCH inhibitors in glial tumors.
Phase I study of RO4929097 with bevacizumab in patients with recurrent malignant glioma.[Pubmed:27826680]
J Neurooncol. 2016 Dec;130(3):571-579.
Antiangiogenic therapies for malignant gliomas often result in transient response, and recurrent disease is characterized by adoption of invasive and hypoxic phenotype. The notch signaling pathway is activated in gliomas, and augments cell migration and hypoxic response. Here we report a clinical study of the combination of bevacizumab and RO4929097, an inhibitor of the notch signaling cascade. A phase I clinical trial was conducted through the Adult Brain Tumor Consortium in subjects with recurrent malignant glioma. Primary objectives were to assess safety and to define the maximum tolerated dose of RO4929097 in combination with bevacizumab. Secondary objectives were to determine overall survival, progression free survival, radiographic response, pharmacokinetic evaluation, and tissue biomarker analysis. Thirteen subjects were enrolled. Of the three subjects treated with the highest dose of RO4929097, one grade 3 toxicity and one grade 2 toxicity were observed. Definitive maximum tolerated dose of RO4929097 in combination with bevacizumab was not identified due to manufacturer's decision to halt drug production. 2 of 12 evaluable subjects demonstrated radiographic response; one subject experienced CR and the second PR. The median overall survival was 10.9 months with a median progression-free survival of 3.7 months. Two subjects remained free of disease progression at 6 months from treatment initiation. PK evaluation did not identify clinically significant drug-drug interactions. All analyzed tissue specimens revealed activation of notch signaling. Combination of RO4929097 and bevacizumab was well-tolerated. Given the compelling scientific rationale, additional studies of antiangiogenic and notch signaling inhibitors should be considered.
Anti-myeloma effect of pharmacological inhibition of Notch/gamma-secretase with RO4929097 is mediated by modulation of tumor microenvironment.[Pubmed:26934342]
Cancer Biol Ther. 2016 May 3;17(5):477-85.
Multiple myeloma (MM), a blood cancer characterized by the uncontrolled proliferation of plasma cells, remains incurable by current therapy. Notch signaling has been implicated in the growth and chemoresistance of various cancer types including MM, and therefore we hypothesized that targeting the Notch pathway could be beneficial for the treatment of this disease. Here, we report an anti-tumor effect of Notch/gamma-secretase inhibitor RO4929097 in a pre-clinical model of MM. We demonstrate that this effect was associated with decreased angiogenesis and significant down-regulation of TGF-beta1. In addition, we also show that treatment with RO4929097 results in decreased number and functional activity of osteoclasts. Taken together, our data indicate that targeting Notch may be considered as a new strategy to be tested for MM therapy.
A phase II study of single-agent RO4929097, a gamma-secretase inhibitor of Notch signaling, in patients with recurrent platinum-resistant epithelial ovarian cancer: A study of the Princess Margaret, Chicago and California phase II consortia.[Pubmed:25769658]
Gynecol Oncol. 2015 May;137(2):216-22.
PURPOSE: A phase II study was performed to evaluate the efficacy and safety of single-agent RO4929097 (a gamma-secretase inhibitor) in patients with recurrent platinum-resistant ovarian cancer. EXPERIMENTAL DESIGN: Women with progressive platinum-resistant ovarian cancer treated with RO4929097 at 20 mg once daily, 3 days on/4 days off each week in a three week cycle. The primary endpoint was progression-free survival (PFS) rate at the end of 4 cycles. Secondary objectives included assessment of the safety of RO4929097 and exploration of molecular correlates of outcome in archival tumor tissue and serum. RESULTS: Of 45 patients enrolled, 40 were evaluable for response. Thirty-seven (82%) patients had high-grade ovarian cancer. No objective responses were observed. Fifteen patients (33%) had stable disease as their best response, with a median duration of 3.1 months. The median PFS for the whole group was 1.3 months (1.2-2.5). Treatment was generally well tolerated with 10% of patients discontinuing treatment due to an adverse event. In high grade serous ovarian cancer patients, the median PFS trended higher when the expression of intracellular Notch (NICD) protein by immunohistochemistry was high versus low (3.3 versus 1.3 months, p=0.09). No clear relationship between circulating angiogenic factors and PFS was found despite a suggestion of an improved outcome with higher baseline VEGFA levels. CONCLUSIONS: RO4929097 has insufficient activity as a single-agent in platinum-resistant ovarian cancer to warrant further study as monotherapy. Future studies are needed to explore the potential for cohort enrichment using NICD expression.


