SDZ SER 082 fumarateSelective 5-HT2B/2C antagonist CAS# 1417343-80-6 |
- MDL-29951
Catalog No.:BCC4059
CAS No.:130798-51-5
- Ro 25-6981 Maleate
Catalog No.:BCC4159
CAS No.:1312991-76-6
- Ro 25-6981
Catalog No.:BCC4158
CAS No.:169274-78-6
- SDZ 220-581 hydrochloride
Catalog No.:BCC4157
CAS No.:179411-93-9
- (+)-MK 801
Catalog No.:BCC1288
CAS No.:70449-94-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

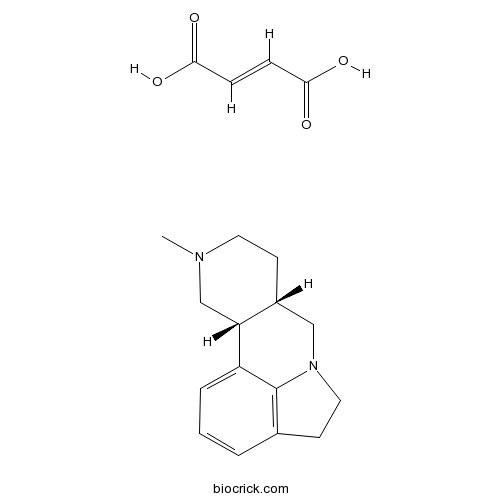
| Cas No. | 1417343-80-6 | SDF | Download SDF |
| PubChem ID | 45073480 | Appearance | Powder |
| Formula | C19H24N2O4 | M.Wt | 344.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| SMILES | CN1CCC2CN3CCC4=C3C(=CC=C4)C2C1.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | DXUZZRDHJMOLTN-MRYVXRNOSA-N | ||
| Standard InChI | InChI=1S/C15H20N2.C4H4O4/c1-16-7-5-12-9-17-8-6-11-3-2-4-13(15(11)17)14(12)10-16;5-3(6)1-2-4(7)8/h2-4,12,14H,5-10H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1+/t12-,14-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective 5-HT2B/2C receptor antagonist with low affinity for 5-HT1A receptors. Inhibits [3H]-mesulergine binding to 5-HT2C receptors with a pKD of 7.8. Inhibits 5-HT2B mediated responses in the rat fundus (pKB = 7.34). |

SDZ SER 082 fumarate Dilution Calculator

SDZ SER 082 fumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9035 mL | 14.5176 mL | 29.0352 mL | 58.0703 mL | 72.5879 mL |
| 5 mM | 0.5807 mL | 2.9035 mL | 5.807 mL | 11.6141 mL | 14.5176 mL |
| 10 mM | 0.2904 mL | 1.4518 mL | 2.9035 mL | 5.807 mL | 7.2588 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5807 mL | 1.1614 mL | 1.4518 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5807 mL | 0.7259 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MM-102
Catalog No.:BCC4551
CAS No.:1417329-24-8
- Exenatide acetate
Catalog No.:BCN8514
CAS No.:141732-76-5
- H-DL-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3175
CAS No.:14173-40-1
- H-Phe(4-Cl)-OH
Catalog No.:BCC3171
CAS No.:14173-39-8
- RI-2
Catalog No.:BCC6424
CAS No.:1417162-36-7
- Faropenem daloxate
Catalog No.:BCC1571
CAS No.:141702-36-5
- Galanin (2-29) (rat)
Catalog No.:BCC5763
CAS No.:141696-11-9
- NPEC-caged-D-AP5
Catalog No.:BCC7895
CAS No.:1416943-27-5
- Thrombin Receptor Activator for Peptide 5 (TRAP-5)
Catalog No.:BCC1025
CAS No.:141685-53-2
- Ivangustin
Catalog No.:BCN3507
CAS No.:14164-59-1
- GR 94800
Catalog No.:BCC5799
CAS No.:141636-65-9
- CU CPT 22
Catalog No.:BCC6320
CAS No.:1416324-85-0
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- Albatrelin A
Catalog No.:BCN7626
CAS No.:1417805-15-2
- Albatrelin C
Catalog No.:BCN7591
CAS No.:1417805-17-4
- Stauntosaponin A
Catalog No.:BCN6966
CAS No.:1417887-91-2
- Endomorphin-2
Catalog No.:BCC5697
CAS No.:141801-26-5
- NMS-873
Catalog No.:BCC4977
CAS No.:1418013-75-8
- LMK 235
Catalog No.:BCC2421
CAS No.:1418033-25-6
- Minaxin C
Catalog No.:BCN7656
CAS No.:1418150-06-7
- EI1
Catalog No.:BCC4044
CAS No.:1418308-27-6
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
Cataleptogenic effect of subtype selective 5-HT receptor antagonists in the rat.[Pubmed:9570468]
Eur J Pharmacol. 1998 Feb 19;343(2-3):201-7.
5-HT receptor antagonists with selectivity for 5-HT1A WAY-100635 (N-[2-[-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohe xanecarboxamide), 5-HT1B GR 127935 (N-[methoxy-3-(4-methyl-1-piperazinyl)phenyl]-2'-methyl-4'(5-methyl-1,2, 4-oxadiazol-3-yl)[1,1-biphenyl]-4-carboxamide x HCl), 5-HT2C SB 200646A (N-(1-methyl-5-indolyl)-N'-(3-pyridyl)urea x HCl) and 5-HT2A (ketanserin, fananserin and MDL 100,151 ((+/-)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-pipe ridinemethanol) receptors were tested for cataleptogenic responses in rats. WAY-100635 (0.1-3 mg/kg, s.c.), ketanserin (0.1-3 mg/kg, s.c.), MDL 100,151 (0.3-3 mg/kg, s.c.) and fananserin (RP 62203; 3 mg/kg, s.c.) induced a significant catalepsy. GR 127935 (1 mg/kg, s.c.), SB 200646A (without effect per se at 10 mg/kg, s.c.) and MDL 100,151 (0.3 mg/kg, s.c.) did not inhibit the cataleptic response to the dopamine D2 receptor antagonist, loxapine (0.3 mg/kg, s.c.). Catalepsy induced by MDL 100,151 (3 mg/kg) was blocked by co-treatment with clozapine, but not by SB 200646A (both at 10 mg/kg, s.c.). Although clozapine displays significant affinity to 5-HT1A, 5-HT1B, 5-HT2A and 5-HT2C receptors, the present results suggest that blockade of these receptors is not responsible for clozapine's anticataleptic activity.
Role of 5-HT2A and 5-HT2C receptor subtypes in the two types of fear generated by the elevated T-maze.[Pubmed:9408213]
Pharmacol Biochem Behav. 1997 Dec;58(4):1051-7.
To study the role of 5-HT2A and 5-HT2C receptor subtypes in anxiety, the behavioral effects of drugs that either block or stimulate these receptors were measured in an animal model of anxiety, the elevated T-maze. One arm of the maze is enclosed by walls and stands perpendicular to the two open arms. Inhibitory (passive) avoidance--representing learned fear--was measured by placing a rat at the end of the enclosed arm and recording the time to leave this arm with the four paws during three consecutive trials. After 30 s, the same animal was placed at the end of one of the open arms and the time to leave this arm with the four paws was recorded. This one-way escape response represents unconditioned fear. The I.P. injection of the preferential 5-HT2C receptor agonists mCPP and TFMPP (0.1-0.8 mg/kg), 25 min before the experimental session enhanced inhibitory avoidance. In contrast, the same drugs either tended to impair (mCPP) or significantly inhibited (TFMPP) one-way escape. The preferential 5-HT2A agonist DOI (0.03-0.3 mg/kg) did not change either inhibitory avoidance or one-way escape. Inhibitory avoidance was impaired by the selective 5-HT2C antagonists SB 200646A (3.0-30 mg/kg) and SDZ SER 082 (0.1-1.0 mg/kg), by the 5-HT2A antagonist SR 46349B (1.0-10.0 mg/kg), and by the mixed 5-HT(2A,2C) antagonist ritanserin (0.3-3.0 mg/kg). However, it was not affected by the selective 5-HT2A antagonist RP 62203 (0.25-4.0 mg/kg). All the 5-HT2 antagonists used were ineffective on one-way escape. Therefore, conditioned fear seems to be tonically facilitated through 5-HT2C receptor stimulation, although the 5-HT2A receptor may also participate in its regulation. Unconditioned fear might be phasically inhibited by 5-HT2C receptor activation.
(+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolo[1,7- bc][2,6]-naphthyridine: a 5-HT2C/2B receptor antagonist with low 5-HT2A receptor affinity.[Pubmed:7837236]
J Med Chem. 1995 Jan 6;38(1):28-33.
The indolonaphthyridine 8 is described as a selective 5-HT2C/2B vs 5-HT2A receptor antagonist. The compound was synthesized in seven steps starting from indoline and isonicotinic acid chloride. The key step is a photocyclization of the indolinyl tetrahydropyridinocarbamic acid ethyl ester 4 to the cis-octahydroindolo[1, 7-bc][2,6]naphthyridinecarbamic acid ethyl ester 5. The synthesis was accomplished by reduction with aluminum hydride and racemic resolution. The indolonaphthyridine 8 exerted the binding profile of a selective 5-HT2C receptor ligand (pKD 7.8) and behaved as an antagonist on the 5-HT-induced accumulation of inositol phosphates in pig choroid plexus cells (pKB 7.13). Compound 8 dose-dependently inhibited the ACTH response to MK-212 in rats and the MK-212-induced hypophagic effect with an ID50 value of 0.3 mg/kg sc. Compound 8 acted as a 5-HT2B receptor antagonist at the rat stomach fundus with a pKB value of 7.34.


