NMS-873VCP/p97 inhibitor,selective and allosteric CAS# 1418013-75-8 |
- DBeQ
Catalog No.:BCC3916
CAS No.:177355-84-9
- Xanthohumol
Catalog No.:BCN5768
CAS No.:569-83-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1418013-75-8 | SDF | Download SDF |
| PubChem ID | 71521142 | Appearance | Powder |
| Formula | C27H28N4O3S2 | M.Wt | 520.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 20.5 mg/mL (39.37 mM; Need ultrasonic and warming) | ||
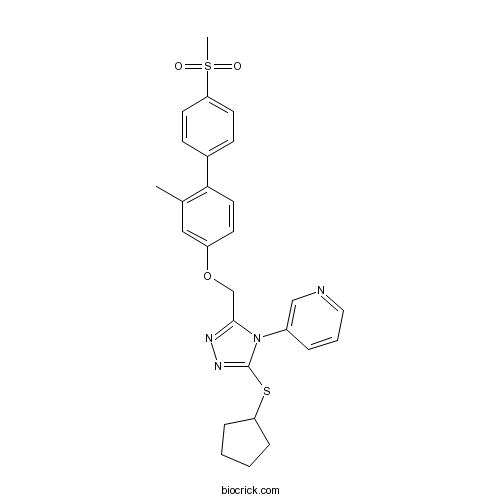
| Chemical Name | 3-[3-cyclopentylsulfanyl-5-[[3-methyl-4-(4-methylsulfonylphenyl)phenoxy]methyl]-1,2,4-triazol-4-yl]pyridine | ||
| SMILES | CC1=C(C=CC(=C1)OCC2=NN=C(N2C3=CN=CC=C3)SC4CCCC4)C5=CC=C(C=C5)S(=O)(=O)C | ||
| Standard InChIKey | UJGTUKMAJVCBIS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H28N4O3S2/c1-19-16-22(11-14-25(19)20-9-12-24(13-10-20)36(2,32)33)34-18-26-29-30-27(35-23-7-3-4-8-23)31(26)21-6-5-15-28-17-21/h5-6,9-17,23H,3-4,7-8,18H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NMS-873 is an allosteric and specific inhibitor of p97 with an IC50 value of 30 nM. | |||||
| Targets | p97 | |||||
| IC50 | 30 nM | |||||

NMS-873 Dilution Calculator

NMS-873 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9206 mL | 9.603 mL | 19.206 mL | 38.412 mL | 48.0151 mL |
| 5 mM | 0.3841 mL | 1.9206 mL | 3.8412 mL | 7.6824 mL | 9.603 mL |
| 10 mM | 0.1921 mL | 0.9603 mL | 1.9206 mL | 3.8412 mL | 4.8015 mL |
| 50 mM | 0.0384 mL | 0.1921 mL | 0.3841 mL | 0.7682 mL | 0.9603 mL |
| 100 mM | 0.0192 mL | 0.096 mL | 0.1921 mL | 0.3841 mL | 0.4802 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NMS-873 is a selective inhibitor of VCP with IC50 value of 30nM [1].
NMS-873 is the most potent and specific VCP inhibitor. It shows similar potency against both wild-type VCP and VCPC522T without affecting the oligomeric state of VCP. NMS-873 is selective against all of the AAA ATPases, HSP90 or some other kinases. The inhibition effect of NMS-873 is potent and somehow selective in a panel of tumor cell lines when its concentration ranges from 0.08μM to 2μM. NMS-873 suppresses cell proliferation in HCT-116 cell line through inducing accumulation of poly-Ub proteins and stabilization of cyclin E and Mcl-1 dose-dependently. Additionally, NMS-873 causes distribution of HCT116 cells, leading a dose and time-dependent increase in the G2 population and in the sub-G1 fraction. Further, NMS-873 shows cell killing activity in hematological tumors as well as a wide variety of solid tumors with IC50 range between 0.08μM and 2μM [1].
References:
[1] Magnaghi P, D'Alessio R, Valsasina B, Avanzi N, Rizzi S, Asa D, Gasparri F, Cozzi L, Cucchi U, Orrenius C, Polucci P, Ballinari D, Perrera C, Leone A, Cervi G, Casale E, Xiao Y, Wong C, Anderson DJ, Galvani A, Donati D, O'Brien T, Jackson PK, Isacchi A. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol. 2013 Sep;9(9):548-56. doi: 10.1038/nchembio.1313. Epub 2013 Jul 28.
- Endomorphin-2
Catalog No.:BCC5697
CAS No.:141801-26-5
- Stauntosaponin A
Catalog No.:BCN6966
CAS No.:1417887-91-2
- Albatrelin C
Catalog No.:BCN7591
CAS No.:1417805-17-4
- Albatrelin A
Catalog No.:BCN7626
CAS No.:1417805-15-2
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- SDZ SER 082 fumarate
Catalog No.:BCC6994
CAS No.:1417343-80-6
- MM-102
Catalog No.:BCC4551
CAS No.:1417329-24-8
- Exenatide acetate
Catalog No.:BCN8514
CAS No.:141732-76-5
- H-DL-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3175
CAS No.:14173-40-1
- H-Phe(4-Cl)-OH
Catalog No.:BCC3171
CAS No.:14173-39-8
- RI-2
Catalog No.:BCC6424
CAS No.:1417162-36-7
- Faropenem daloxate
Catalog No.:BCC1571
CAS No.:141702-36-5
- LMK 235
Catalog No.:BCC2421
CAS No.:1418033-25-6
- Minaxin C
Catalog No.:BCN7656
CAS No.:1418150-06-7
- EI1
Catalog No.:BCC4044
CAS No.:1418308-27-6
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- 4-Hydroxyphenylacetonitrile
Catalog No.:BCN6905
CAS No.:14191-95-8
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Ginsenoside Rg3
Catalog No.:BCN1068
CAS No.:14197-60-5
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
Identification of NMS-873, an allosteric and specific p97 inhibitor, as a broad antiviral against both influenza A and B viruses.[Pubmed:30930289]
Eur J Pharm Sci. 2019 Mar 28;133:86-94.
Influenza virus infection causes substantial morbidity and mortality worldwide. The limited efficacy of oseltamivir in delayed treatment, coupled with the increasing incidences of oseltamivir-resistant strains, calls for next-generation of antiviral drugs. In this study, we discovered NMS-873, an allosteric and specific p97 inhibitor, as a broad-spectrum influenza antiviral through forward chemical genomics screening. NMS-873 shows potent antiviral activity with low-nanomolar EC50s against multiple human influenza A and B viruses, including adamantine-, oseltamivir-, or double resistant strains. Our data further showed that silencing of p97 via siRNA or inhibiting p97 by NMS-873 both inhibited virus replication and retained viral ribonucleoproteins (vRNPs) in the nucleus, confirming p97 is the drug target. Mechanistic studies have shown that the nuclear retention of vRNP with NMS-873 treatment is a combined result of two effects: the reduced viral M1 protein level (indirect effect), and the disruption of p97-NP interactions (direct effect). Taken together, our results suggest that p97 could be a novel antiviral target and its inhibitor, NMS-873, is a promising antiviral drug candidate.
Adapted ATPase domain communication overcomes the cytotoxicity of p97 inhibitors.[Pubmed:30381397]
J Biol Chem. 2018 Dec 28;293(52):20169-20180.
The AAA(+) ATPase p97 regulates ubiquitin-dependent protein homeostasis and has been pursued as a cancer drug target. The ATP-competitive inhibitor CB-5083 and allosteric inhibitor NMS-873 are the most advanced p97 inhibitors described to date. Previous studies have reported that their cytotoxicity can be readily overcome and involves single p97 mutations in the linker between the D1 and D2 ATPase domains and within D2. We report here that the proline 472 to leucine (P472L) mutation, in the D1-D2 linker and identified in CB-5083-resistant cells, desensitizes p97 to both inhibitor classes. This mutation does not disrupt the distinct D2-binding sites of the inhibitors. Instead, P472L changes ATPase domain communication within the p97 hexamer. P472L enhances cooperative D2 ATP binding and hydrolysis. This mechanism alters the function of the D1-D2 linker in the control of D2 activity involving the ATP-bound state of D1. Although increased D2 activity is sufficient to desensitize the P472L mutant to NMS-873, the mutant's desensitization to CB-5083 also requires D1 ATPase domain function. Our study highlights the remarkable adaptability of p97 ATPase domain communication that enables escape from mechanistically distinct classes of cytotoxic p97 inhibitors.
Essential function of VCP/p97 in infection cycle of the nucleopolyhedrovirus AcMNPV in Spodoptera frugiperda Sf9 cells.[Pubmed:29890203]
Virus Res. 2018 Jul 15;253:68-76.
The protein VCP/p97 (also named CDC48 and TER94) belongs to a type II subfamily of the AAA+ATPases and controls cellular proteostasis by acting upstream of proteasomes in the ubiquitin-proteasome protein degradation pathway. The function of VCP/p97 in the baculovirus infection cycle in insect cells remains unknown. Here, we identified VCP/p97 in the fall armyworm Spodoptera frugiperda (Sf9) cells and analyzed the replication of the Autographa californica multiple nucleopolyhedrovirus, AcMNPV, in Sf9 cells in which the VCP/p97 function was inhibited. The specific allosteric inhibitor of the VCP/p97 ATPase activity, NMS-873, did not deplete VCP/p97 in infected cells but caused a dose-dependent inhibition of viral DNA synthesis and efficiently suppressed expression of viral proteins and production of budded virions. NMS-873 caused accumulation of ubiquitinated proteins in a manner similar to the inhibitor of proteasome activity, Bortezomib. This suggests the essential function of VCP/p97 in the baculovirus infection cycle might be associated, at least in part, with the ubiquitin-proteasome system.
Interleukin-6 induced overexpression of valosin-containing protein (VCP)/p97 is associated with androgen-independent prostate cancer (AIPC) progression.[Pubmed:29693262]
J Cell Physiol. 2018 Oct;233(10):7148-7164.
Though Androgen deprivation therapy (ADT) is effective initially, numerous patients become resistant to it and develop castration resistant PCa (CRPC). Cytokines promotes ligand independent activation of AR. Interleukin-6 (IL-6) levels are elevated in CRPC patients and regulate AR activity. However, progression to CRPC is not fully understood. In this study, we analyzed differential protein expression in LNCaP cells treated with IL-6 using proteomics. Results revealed altered expression of 27 proteins and Valosin-containing protein (VCP)/p97 plays a predominant role in co-regulation of altered proteins. Interestingly, IL-6 induced VCP expression through Pim-1 via STAT3 is AR independent there by suggesting a role for VCP in CRPC. Transfection of LNCaP cells for VCP overexpression showed an increased cell proliferation, migration, and invasion where as its inhibition by NMS-873 showed the reverse effect causing cell death. Mechanistic studies demonstrate that cell death occurs due to apoptosis by endoplasmic reticulum (ER) stress, elevated cell cycle inhibitors p21, p27kip1, and active PARP and reduced Bcl-2. VCP promotes cell invasion and migration by altering E-cadherin and Vimentin levels inversely triggering EMT of PCa cells. VCP immunostaining revealed no staining in BPH but strong staining in PCa. This study determines VCP may play an important role in progression to CRPC and it can be a favorable target with to develop new therapies to treat ADT resistant prostate cancer.
The host ubiquitin-dependent segregase VCP/p97 is required for the onset of human cytomegalovirus replication.[Pubmed:28494016]
PLoS Pathog. 2017 May 11;13(5):e1006329.
The human cytomegalovirus major immediate early proteins IE1 and IE2 are critical drivers of virus replication and are considered pivotal in determining the balance between productive and latent infection. IE1 and IE2 are derived from the same primary transcript by alternative splicing and regulation of their expression likely involves a complex interplay between cellular and viral factors. Here we show that knockdown of the host ubiquitin-dependent segregase VCP/p97, results in loss of IE2 expression, subsequent suppression of early and late gene expression and, ultimately, failure in virus replication. RNAseq analysis showed increased levels of IE1 splicing, with a corresponding decrease in IE2 splicing following VCP knockdown. Global analysis of viral transcription showed the expression of a subset of viral genes is not reduced despite the loss of IE2 expression, including UL112/113. Furthermore, Immunofluorescence studies demonstrated that VCP strongly colocalised with the viral replication compartments in the nucleus. Finally, we show that NMS-873, a small molecule inhibitor of VCP, is a potent HCMV antiviral with potential as a novel host targeting therapeutic for HCMV infection.
Opposing roles of Nfkb2 gene products p100 and p52 in the regulation of breast cancer stem cells.[Pubmed:28190248]
Breast Cancer Res Treat. 2017 Apr;162(3):465-477.
PURPOSE: Nuclear factor-kappa B (NF-kappaB) signalling has been shown to regulate properties of breast cancer stem cells. However, the specific contribution of the non-canonical NF-kappaB pathway, components of which are elevated in aggressive breast cancer has not been addressed. METHODS: Through shRNA silencing of the Nfkb2 gene, the role of p100/p52 in 4T1 and N202.1A cell lines were assessed by NF-kappaB reporter, invasion, tumoursphere and orthotopic transplantation assays. The processing of p100 into p52 was also inhibited with a p97 ATPase inhibitor, NMS-873, and its effects on tumoursphere formation was assessed. RESULTS: Knockdown of Nfkb2 led to opposing changes in NF-kappaB-dependent transcription. NF-kappaB activity was elevated in 4T1 cells and this resulted in increased motility, cancer stem cell (CSC) activity and tumourigenicity in vivo. Conversely, depletion of Nfkb2 in N202.1a cells decreased NF-kappaB activity, CSC properties and tumourigenicity in vivo. By selectively overexpressing the p52 subunit in Nfkb2 depleted cells, we found that the increased malignancy in 4T1 cells could not be reverted in the presence of p52, whereas the decreased tumourigenicity of N202.1a cells could be rescued by p52. These results indicate that p100 and its subunit p52 have opposing effects on breast CSC activity. Accordingly, inhibition of an upstream regulator of p100 processing was effective in reducing tumoursphere formation of N202.1A and SKBR3 (ErbB2 (HIGH)) cells without aggravating that of 4T1 and MDA-MB-231 (ErbB2(LOW)) cells. CONCLUSION: These findings indicate that inhibiting the processing of p100 may be a potential therapeutic strategy to suppress CSC activity in a subset of breast tumours.
Dendrimer-Based Selective Proteostasis-Inhibition Strategy to Control NSCLC Growth and Progression.[Pubmed:27434122]
PLoS One. 2016 Jul 19;11(7):e0158507.
Elevated valosin containing protein (VCP/p97) levels promote the progression of non-small cell lung carcinoma (NSCLC). Although many VCP inhibitors are available, most of these therapeutic compounds have low specificity for targeted tumor cell delivery. Hence, the primary aim of this study was to evaluate the in vitro efficacy of dendrimer-encapsulated potent VCP-inhibitor drug in controlling non-small cell lung carcinoma (NSCLC) progression. The VCP inhibitor(s) (either in their pure form or encapsulated in generation-4 PAMAM-dendrimer with hydroxyl surface) were tested for their in vitro efficacy in modulating H1299 (NSCLC cells) proliferation, migration, invasion, apoptosis and cell cycle progression. Our results show that VCP inhibition by DBeQ was significantly more potent than NMS-873 as evident by decreased cell proliferation (p<0.0001, MTT-assay) and migration (p<0.05; scratch-assay), and increased apoptosis (p<0.05; caspase-3/7-assay) as compared to untreated control cells. Next, we found that dendrimer-encapsulated DBeQ (DDNDBeQ) treatment increased ubiquitinated-protein accumulation in soluble protein-fraction (immunoblotting) of H1299 cells as compared to DDN-control, implying the effectiveness of DBeQ in proteostasis-inhibition. We verified by immunostaining that DDNDBeQ treatment increases accumulation of ubiquitinated-proteins that co-localizes with an ER-marker, KDEL. We observed that proteostasis-inhibition with DDNDBeQ, significantly decreased cell migration rate (scratch-assay and transwell-invasion) as compared to the control-DDN treatment (p<0.05). Moreover, DDNDBeQ treatment showed a significant decrease in cell proliferation (p<0.01, MTT-assay) and increased caspase-3/7 mediated apoptotic cell death (p<0.05) as compared to DDN-control. This was further verified by cell cycle analysis (propidium-iodide-staining) that demonstrated significant cell cycle arrest in the G2/M-phase (p<0.001) by DDNDBeQ treatment as compared to control-DDN. Moreover, we confirmed by clonogenic-assay that DDNDBeQ treatment significantly (p<0.001) inhibits H1299 colony-formation as compared to control/DDN. Overall, encapsulation of potent VCP-inhibitor DBeQ into a dendrimer allows selective VCP-mediated proteostasis-inhibition for controlling NSCLC-tumor growth and progression to allow tumor-targeted sustained drug delivery.
Bifunctional alkylating agent-mediated MGMT-DNA cross-linking and its proteolytic cleavage in 16HBE cells.[Pubmed:27342729]
Toxicol Appl Pharmacol. 2016 Aug 15;305:267-273.
Nitrogen mustard (NM), a bifunctional alkylating agent (BAA), contains two alkyl arms and can act as a cross-linking bridge between DNA and protein to form a DNA-protein cross-link (DPC). O(6)-methylguanine-DNA methyltransferase (MGMT), a DNA repair enzyme for alkyl adducts removal, is found to enhance cell sensitivity to BAAs and to promote damage, possibly due to its stable covalent cross-linking with DNA mediated by BAAs. To investigate MGMT-DNA cross-link (mDPC) formation and its possible dual roles in NM exposure, human bronchial epithelial cell line 16HBE was subjected to different concentrations of HN2, a kind of NM, and we found mDPC was induced by HN2 in a concentration-dependent manner, but the mRNA and total protein of MGMT were suppressed. As early as 1h after HN2 treatment, high mDPC was achieved and the level maintained for up to 24h. Quick total DPC (tDPC) and gamma-H2AX accumulation were observed. To evaluate the effect of newly predicted protease DVC1 on DPC cleavage, we applied siRNA of MGMT and DVC1, MG132 (proteasome inhibitor), and NMS-873 (p97 inhibitor) and found that proteolysis plays a role. DVC1 was proven to be more important in the cleavage of mDPC than tDPC in a p97-dependent manner. HN2 exposure induced DVC1 upregulation, which was at least partially contributed to MGMT cleavage by proteolysis because HN2-induced mDPC level and DNA damage was closely related with DVC1 expression. Homologous recombination (HR) was also activated. Our findings demonstrated that MGMT might turn into a DNA damage promoter by forming DPC when exposed to HN2. Proteolysis, especially DVC1, plays a crucial role in mDPC repair.
p97 Composition Changes Caused by Allosteric Inhibition Are Suppressed by an On-Target Mechanism that Increases the Enzyme's ATPase Activity.[Pubmed:27105284]
Cell Chem Biol. 2016 Apr 21;23(4):517-28.
The AAA ATPase p97/VCP regulates protein homeostasis using a diverse repertoire of cofactors to fulfill its biological functions. Here we use the allosteric p97 inhibitor NMS-873 to analyze its effects on enzyme composition and the ability of cells to adapt to its cytotoxicity. We found that p97 inhibition changes steady state cofactor-p97 composition, leading to the enrichment of a subset of its cofactors and polyubiquitin bound to p97. We isolated cells specifically insensitive to NMS-873 and identified a new mutation (A530T) in p97. A530T is sufficient to overcome the cytotoxicity of NMS-873 and alleviates p97 composition changes caused by the molecule but not other p97 inhibitors. This mutation does not affect NMS-873 binding but increases p97 catalytic efficiency through altered ATP and ADP binding. Collectively, these findings identify cofactor-p97 interactions sensitive to p97 inhibition and reveal a new on-target mechanism to suppress the cytotoxicity of NMS-873.
Specific inhibition of p97/VCP ATPase and kinetic analysis demonstrate interaction between D1 and D2 ATPase domains.[Pubmed:24878061]
J Mol Biol. 2014 Jul 29;426(15):2886-99.
The p97 AAA (ATPase associated with diverse cellular activities), also called VCP (valosin-containing protein), is an important therapeutic target for cancer and neurodegenerative diseases. p97 forms a hexamer composed of two AAA domains (D1 and D2) that form two stacked rings and an N-terminal domain that binds numerous cofactor proteins. The interplay between the three domains in p97 is complex, and a deeper biochemical understanding is needed in order to design selective p97 inhibitors as therapeutic agents. It is clear that the D2 ATPase domain hydrolyzes ATP in vitro, but whether D1 contributes to ATPase activity is controversial. Here, we use Walker A and B mutants to demonstrate that D1 is capable of hydrolyzing ATP and show for the first time that nucleotide binding in the D2 domain increases the catalytic efficiency (kcat/Km) of D1 ATP hydrolysis 280-fold, by increasing kcat 7-fold and decreasing Km about 40-fold. We further show that an ND1 construct lacking D2 but including the linker between D1 and D2 is catalytically active, resolving a conflict in the literature. Applying enzymatic observations to small-molecule inhibitors, we show that four p97 inhibitors (DBeQ, ML240, ML241, and NMS-873) have differential responses to Walker A and B mutations, to disease-causing IBMPFD mutations, and to the presence of the N domain binding cofactor protein p47. These differential effects provide the first evidence that p97 cofactors and disease mutations can alter p97 inhibitor potency and suggest the possibility of developing context-dependent inhibitors of p97.
Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death.[Pubmed:23892893]
Nat Chem Biol. 2013 Sep;9(9):548-56.
VCP (also known as p97 or Cdc48p in yeast) is an AAA(+) ATPase regulating endoplasmic reticulum-associated degradation. After high-throughput screening, we developed compounds that inhibit VCP via different mechanisms, including covalent modification of an active site cysteine and a new allosteric mechanism. Using photoaffinity labeling, structural analysis and mutagenesis, we mapped the binding site of allosteric inhibitors to a region spanning the D1 and D2 domains of adjacent protomers encompassing elements important for nucleotide-state sensing and ATP hydrolysis. These compounds induced an increased affinity for nucleotides. Interference with nucleotide turnover in individual subunits and distortion of interprotomer communication cooperated to impair VCP enzymatic activity. Chemical expansion of this allosteric class identified NMS-873, the most potent and specific VCP inhibitor described to date, which activated the unfolded protein response, interfered with autophagy and induced cancer cell death. The consistent pattern of cancer cell killing by covalent and allosteric inhibitors provided critical validation of VCP as a cancer target.


