EI1EZH2 inhibitor CAS# 1418308-27-6 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1418308-27-6 | SDF | Download SDF |
| PubChem ID | 72199293 | Appearance | Powder |
| Formula | C23H26N4O2 | M.Wt | 390.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 7.69 mg/mL (19.69 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 6-cyano-N-[(4,6-dimethyl-2-oxo-1H-pyridin-3-yl)methyl]-1-pentan-3-ylindole-4-carboxamide | ||
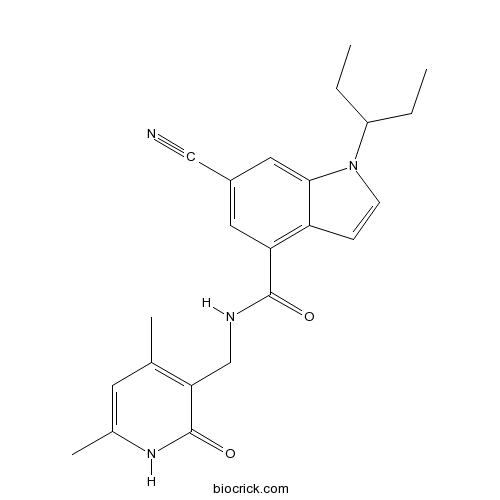
| SMILES | CCC(CC)N1C=CC2=C(C=C(C=C21)C#N)C(=O)NCC3=C(C=C(NC3=O)C)C | ||
| Standard InChIKey | PFHDWRIVDDIFRP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H26N4O2/c1-5-17(6-2)27-8-7-18-19(10-16(12-24)11-21(18)27)22(28)25-13-20-14(3)9-15(4)26-23(20)29/h7-11,17H,5-6,13H2,1-4H3,(H,25,28)(H,26,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

EI1 Dilution Calculator

EI1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.561 mL | 12.8048 mL | 25.6095 mL | 51.219 mL | 64.0238 mL |
| 5 mM | 0.5122 mL | 2.561 mL | 5.1219 mL | 10.2438 mL | 12.8048 mL |
| 10 mM | 0.2561 mL | 1.2805 mL | 2.561 mL | 5.1219 mL | 6.4024 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0244 mL | 1.2805 mL |
| 100 mM | 0.0256 mL | 0.128 mL | 0.2561 mL | 0.5122 mL | 0.6402 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: A potent and selective suppressor of Ezh2 with IC50 values of 15 nM and 13 nM for wild type Ezh2 and Ezh2 Y641F mutant, respectively
EI1 performs as a highly potent inhibitor of Ezh2 which is named enhancer of zeste homolog 2. Mutation and over-expression of Ezh2 has been linked to the development of cancer. Since Ezh2 is upregulated in multiple cancers, it seems to play a crucial role in the regulation of tumor angiogenesis and has been considered as a potential target in anticancer therapy. Ezh2 stimulates cancer development by inhibiting tumor-suppressing genes. Suppressing Ezh2 activity may therefore inhibit tumor growth. [1]
In vitro: Based on studies from DLBCL cells, it was shown that EI1 suppressed cellular H3K27 methylation and activated expression of Ezh2 target gene - p16. EI1 also blocked H3K27 methylation and cell proliferation in mouse embryonic fibroblasts. In addition, EI1 potently and selectively inhibited the growth of DLBCL cells carrying Ezh2 mutation, and therefore resulted in cell cycle arrest and apoptosis. [1]
In vivo: So far, no in vivo study has been conducted.
Clinical trial: So far, no clinical trial has been conducted.
Reference:
[1]Qia W, Chan HM, Teng L, Li L, Chuai S, Zhang RP, Zeng J, Li M, Fan H, Lin Y, Gu J, Ardayfiob O, Zhang JH, Yan X, Fang J, Mi Y, Zhang M, Zhou T, Feng G, Chen ZJ, Li G, Yang T, Zhao K, Liu X, Yu Z, Lu CX, Atadja P and Li E. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci. 2012 Dec; 109(52): 213605.
- Minaxin C
Catalog No.:BCN7656
CAS No.:1418150-06-7
- LMK 235
Catalog No.:BCC2421
CAS No.:1418033-25-6
- NMS-873
Catalog No.:BCC4977
CAS No.:1418013-75-8
- Endomorphin-2
Catalog No.:BCC5697
CAS No.:141801-26-5
- Stauntosaponin A
Catalog No.:BCN6966
CAS No.:1417887-91-2
- Albatrelin C
Catalog No.:BCN7591
CAS No.:1417805-17-4
- Albatrelin A
Catalog No.:BCN7626
CAS No.:1417805-15-2
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- SDZ SER 082 fumarate
Catalog No.:BCC6994
CAS No.:1417343-80-6
- MM-102
Catalog No.:BCC4551
CAS No.:1417329-24-8
- Exenatide acetate
Catalog No.:BCN8514
CAS No.:141732-76-5
- H-DL-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3175
CAS No.:14173-40-1
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- 4-Hydroxyphenylacetonitrile
Catalog No.:BCN6905
CAS No.:14191-95-8
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Ginsenoside Rg3
Catalog No.:BCN1068
CAS No.:14197-60-5
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
- Emetine Hydrochloride
Catalog No.:BCN2478
CAS No.:14198-59-5
- Methyl 4-Hydroxyphenylacetate
Catalog No.:BCN1571
CAS No.:14199-15-6
- NSC 625987
Catalog No.:BCC7269
CAS No.:141992-47-4
Use of molecular markers to compare Fusarium verticillioides pathogenic strains isolated from plants and humans.[Pubmed:24065642]
Genet Mol Res. 2013 Aug 12;12(3):2863-75.
Fusarium verticillioides is a pathogen of agriculturally important crops, especially maize. It is considered one of the most important pathogens responsible for fumonisin contamination of food products, which causes severe, chronic, and acute intoxication in humans and animals. Moreover, it is recognized as a cause of localized infections in immunocompetent patients and disseminated infections among severely immunosuppressed patients. Several molecular tools have been used to analyze the intraspecific variability of fungi. The objective of this study was to use molecular markers to compare pathogenic isolates of F. verticillioides and isolates of the same species obtained from clinical samples of patients with Fusarium mycoses. The molecular markers that we used were inter-simple sequence repeat markers (primers GTG5 and GACA4), intron splice site primer (primer EI1), random amplified polymorphic DNA marker (primer OPW-6), and restriction fragment length polymorphism-internal transcribed spacer (ITS) from rDNA. From the data obtained, clusters were generated based on the UPGMA clustering method. The amplification products obtained using primers ITS4 and ITS5 and loci ITS1-5.8-ITS2 of the rDNA yielded fragments of approximately 600 bp for all the isolates. Digestion of the ITS region fragment using restriction enzymes such as EcoRI, DraI, BshI, AluI, HaeIII, HinfI, MspI, and PstI did not permit differentiation among pathogenic and clinical isolates. The inter-simple sequence repeat, intron splice site primer, and random amplified polymorphic DNA markers presented high genetic homogeneity among clinical isolates in contrast to the high variability found among the phytopathogenic isolates of F. verticillioides.
EZH2 inhibitors transcriptionally upregulate cytotoxic autophagy and cytoprotective unfolded protein response in human colorectal cancer cells.[Pubmed:27648357]
Am J Cancer Res. 2016 Aug 1;6(8):1661-80. eCollection 2016.
Enhancer of zeste homolog 2 (EZH2) has been emerged as novel anticancer target. Various EZH2 small-molecule inhibitors have been developed in recent years. A major class of EZH2 inhibitors are S-adenosyl-L-methionine (SAM)-competitive inhibitors, such as EPZ005687, EI1, GSK126, UNC1999 and GSK343. Autophagy, a physiological process of self-digestion, is involved in the turnover of proteins or intracellular organelles. It can serve as cytoprotective or cytotoxic function in cancer. Our previous study has found that UNC1999 and GSK343 are potent autophagy inducers. In this study, the underlying molecular mechanisms were further investigated. Our results showed that UNC1999 and GSK343 transcriptionally upregulated autophagy of human colorectal cancer (CRC) cells through inducing LC3B gene expression. Besides, UNC1999/GSK343-induced autophagy was partially dependent on ATG7 but independent to EZH2 inhibition. Microarray and PCR array analyses identified that UNC1999 and GSK343 also induced endoplasmic reticulum (ER) stress and unfolded protein response (UPR). UNC1999/GSK343-induced ER stress/UPR contributed to the survival of cancer cells, which was opposite to UNC1999/GSK343-induced autophagy that promoted cell death.
Sonoelastography as method for preliminary evaluation of uterine cervix to predict success of induction of labor.[Pubmed:24247111]
Fetal Diagn Ther. 2014;35(1):57-61.
INTRODUCTION: Induction of labor is a useful practice to solve many obstetric situations but has a large impact on the health of women and their babies and therefore needs to be clearly justified clinically. AIM: To determine the sensitivity of sonoelastography in the evaluation of the cervix to predict the success of induction. MATERIALS AND METHODS: We enrolled 53 subjects preparing for induction of labor. Transvaginal evaluation of cervical length and a sonoelastogram were performed. We preliminarily classified the sonoelastograms into five elastography index (EI) categories and examined the different distribution of cesarean or spontaneous deliveries in various subgroups of EI by chi(2) test and multivariate analysis by logistic regression. RESULTS: Statistical analysis revealed a significant difference of prevalence of spontaneous delivery (EI1-3 82.75%, EI4-5 45.8%) versus cesarean section (EI1-3 17.25%, EI4-5 54.16%) (p = 0.0072). The diagnostic validity of EI was evaluated using the receiver operating characteristic curve and cut-off of the predictive value was EI3. DISCUSSION: The results of our study indicate that sonoelastography is an innovative technique that could allow a more objective preliminary evaluation of the cervix before inducing labor, however further studies with a larger number of subjects and a standardization of image acquisition are necessary.
Previous exercise training increases levels of PPAR-alpha in long-term post-myocardial infarction in rats, which is correlated with better inflammatory response.[Pubmed:27074178]
Clinics (Sao Paulo). 2016 Mar;71(3):163-8.
OBJECTIVE: Exercise is a protective factor for cardiovascular morbidity and mortality, with unclear mechanisms. Changing the myocardial metabolism causes harmful consequences for heart function and exercise contributes to metabolic adjustment modulation. Peroxisome proliferator-activated receptors (PPARs) are also myocardium metabolism regulators capable of decreasing the inflammatory response. We hypothesized that PPAR-alpha is involved in the beneficial effects of previous exercise on myocardial infarction (MI) and cardiac function, changing the expression of metabolic and inflammatory response regulators and reducing myocardial apoptosis, which partially explains the better outcome. METHODS AND RESULTS: Exercised rats engaged in swimming sessions for 60 min/day, 5 days/week, for 8 weeks. Both the exercised rats and sedentary rats were randomized to MI surgery and followed for 1 week (EI1 or SI1) or 4 weeks (EI4 or SI4) of healing or to sham groups. Echocardiography was employed to detect left ventricular function and the infarct size. Additionally, the TUNEL technique was used to assess apoptosis and immunohistochemistry was used to quantitatively analyze the PPAR-alpha, TNF-alpha and NF-kappaB antigens in the infarcted and non-infarcted myocardium. MI-related mortality was higher in SI4 than in EI4 (25% vs 12%), without a difference in MI size. SI4 exhibited a lower shortening fraction than EI4 did (24% vs 35%) and a higher apoptosis/area rate (3.97+/-0.61 vs 1.90+/-1.82) in infarcted areas (both p=0.001). Immunohistochemistry also revealed higher TNF-alpha levels in SI1 than in EI1 (9.59 vs 4.09, p<0.001) in infarcted areas. In non-infarcted areas, EI4 showed higher levels of TNF-alpha and positive correlations between PPAR-alpha and NF-kappaB (r=0.75, p=0.02), in contrast to SI4 (r=0.05, p=0.87). CONCLUSION: Previously exercised animals had better long-term ventricular function post-MI, in addition to lower levels of local inflammatory markers and less myocardial apoptosis, which seemed to be related to the presence of PPAR-alpha.
Development of secondary mutations in wild-type and mutant EZH2 alleles cooperates to confer resistance to EZH2 inhibitors.[Pubmed:25893294]
Oncogene. 2016 Feb 4;35(5):558-66.
The histone methyltransferase Enhancer of Zeste Homolog 2 (EZH2) is frequently dysregulated in cancers, and gain-of-function (GOF) EZH2 mutations have been identified in non-Hodgkin lymphomas. Small-molecule inhibitors against EZH2 demonstrated anti-tumor activity in EZH2-mutated lymphomas and entered clinical trials. Here, we developed models of acquired resistance to EZH2 inhibitor EI1 with EZH2-mutated lymphoma cells. Resistance was generated by secondary mutations in both wild-type (WT) and GOF Y641N EZH2 alleles. These EZH2 mutants retained the substrate specificity of their predecessor complexes but became refractory to biochemical inhibition by EZH2 inhibitors. Resistant cells were able to maintain a high level of H3K27Me3 in the presence of inhibitors. Interestingly, mutation of EZH2 WT alone generated an intermediate resistance phenotype, which is consistent with a previously proposed model of cooperation between EZH2 WT and Y641N mutants to promote tumorigenesis. In addition, the findings presented here have implications for the clinical translation of EZH2 inhibitors and underscore the need to develop novel EZH2 inhibitors to target potential resistance emerging in clinical settings.


