LMK 235HDAC4/HDAC5 inhibitor CAS# 1418033-25-6 |
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

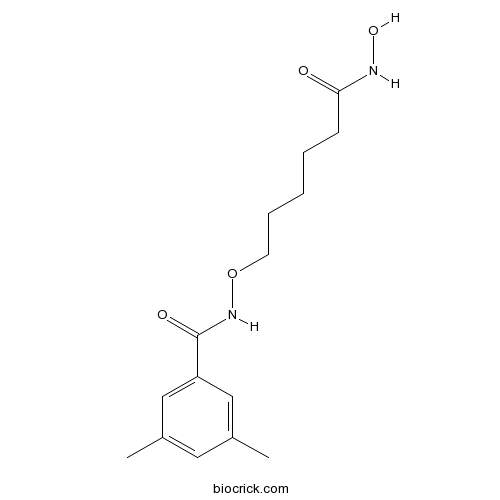
| Cas No. | 1418033-25-6 | SDF | Download SDF |
| PubChem ID | 71520717 | Appearance | Powder |
| Formula | C15H22N2O4 | M.Wt | 294.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (101.92 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[6-(hydroxyamino)-6-oxohexoxy]-3,5-dimethylbenzamide | ||
| SMILES | CC1=CC(=CC(=C1)C(=O)NOCCCCCC(=O)NO)C | ||
| Standard InChIKey | VRYZCEONIWEUAV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H22N2O4/c1-11-8-12(2)10-13(9-11)15(19)17-21-7-5-3-4-6-14(18)16-20/h8-10,20H,3-7H2,1-2H3,(H,16,18)(H,17,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective histone deacetylase (HDAC) 4 and HDAC5 inhibitor (IC50 values are 4.22, 11.9, 55.7, 320, 852, 881, and 1278 nM for HDAC 5, 4, 6, 1, 11, 2, and 8, respectively). Demonstrates activity against chemoresistant cancer cell lines in an MTT assay for cytotoxicity using human ovarian cancer cell lines A2780 and cisplatin resistant A2780CisR (IC50 = 0.49 and 0.32 μM respectively). |

LMK 235 Dilution Calculator

LMK 235 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3973 mL | 16.9866 mL | 33.9732 mL | 67.9463 mL | 84.9329 mL |
| 5 mM | 0.6795 mL | 3.3973 mL | 6.7946 mL | 13.5893 mL | 16.9866 mL |
| 10 mM | 0.3397 mL | 1.6987 mL | 3.3973 mL | 6.7946 mL | 8.4933 mL |
| 50 mM | 0.0679 mL | 0.3397 mL | 0.6795 mL | 1.3589 mL | 1.6987 mL |
| 100 mM | 0.034 mL | 0.1699 mL | 0.3397 mL | 0.6795 mL | 0.8493 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description:
IC50: cytotoxicity IC50 (490 nm for A2780; 320 nm for A2780 CisR); HDAC inhibition (650 nM for A2780; 320 nm for A2780 CisR)
Histone deacetylases (EC 3.5.1.98, HDAC) are a class of enzymes that remove acetyl groups (O=C-CH3) from an ε-N-acetyl lysine amino acid on a histone, allowing the histones to wrap the DNA more tightly. Histone deacetylase inhibitors (HDIs) have a long history of use in psychiatry and neurology as mood stabilizers and anti-epileptics, for example, valproic acid. In more recent times, HDIs are being studied as a mitigator or treatment for neurodegenerative diseases.
In vitro: LMK235 showed similar effects compared to vorinostat on inhibition of cellular HDACs in a pan-HDAC assay but enhanced cytotoxic effects against the human cancer cell lines A2780, Cal27, Kyse510, and MDA-MB231. LMK235 shows nanomolar inhibition of HDAC4 and HDAC5, whereas vorinostat and TSA inhibit HDAC4 and HDAC5 in the higher micromolar range. In contrast to vorinostat, LMK235 showed a novel HDAC isoform selectivity profile with preference for HDAC4 and HDAC5, which are inhibited with low nanomolar IC50 values [1].
In vivo: In a mouse in-vivo study, synergistic inhibition was demonstrated for LMK235 and Cytarabine in proliferation assays and in colony formation assays. These findings demonstrate that in vivo RNAi screening for therapeutic efficacy is feasible. HDAC4 might be an important target to enhance efficacy of anti-leukemic drugs [2].
Clinical trial: LMK235 is currently in the preclinical development and no clinical trial is ongoing.
References:
[1] Marek L, Hamacher A, Hansen FK, Kuna K, Gohlke H, Kassack MU, Kurz T. Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. J Med Chem. 2013;56(2):427-36.
[2] Stephanie Lettermann, Konstantin Agelopoulos, Christian Rohde, Beate Lindtner, Linda Marek, Georg Hempel, Matthias Stelljes, Wolfgang E. Berdel, Thomas Kurz and Carsten Müller-Tidow. In Vivo RNAi Screening Identifies HDAC4 As a Mediator Of Chemoresistance In Acute Myeloid Leukemia. 55th ASH Annual Meeting and Exposition (2013) (https://ash.confex.com/ash/2013/webprogram/Paper60036.html)
- NMS-873
Catalog No.:BCC4977
CAS No.:1418013-75-8
- Endomorphin-2
Catalog No.:BCC5697
CAS No.:141801-26-5
- Stauntosaponin A
Catalog No.:BCN6966
CAS No.:1417887-91-2
- Albatrelin C
Catalog No.:BCN7591
CAS No.:1417805-17-4
- Albatrelin A
Catalog No.:BCN7626
CAS No.:1417805-15-2
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- SDZ SER 082 fumarate
Catalog No.:BCC6994
CAS No.:1417343-80-6
- MM-102
Catalog No.:BCC4551
CAS No.:1417329-24-8
- Exenatide acetate
Catalog No.:BCN8514
CAS No.:141732-76-5
- H-DL-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3175
CAS No.:14173-40-1
- H-Phe(4-Cl)-OH
Catalog No.:BCC3171
CAS No.:14173-39-8
- RI-2
Catalog No.:BCC6424
CAS No.:1417162-36-7
- Minaxin C
Catalog No.:BCN7656
CAS No.:1418150-06-7
- EI1
Catalog No.:BCC4044
CAS No.:1418308-27-6
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- 4-Hydroxyphenylacetonitrile
Catalog No.:BCN6905
CAS No.:14191-95-8
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Ginsenoside Rg3
Catalog No.:BCN1068
CAS No.:14197-60-5
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
- Emetine Hydrochloride
Catalog No.:BCN2478
CAS No.:14198-59-5
HDAC5, a potential therapeutic target and prognostic biomarker, promotes proliferation, invasion and migration in human breast cancer.[Pubmed:27177225]
Oncotarget. 2016 Jun 21;7(25):37966-37978.
PURPOSE: Histone deacetylase 5 (HDAC5) is an important protein in neural and cardiac diseases and a potential drug target. However, little is known regarding the specific role of HDAC5 in breast cancer (BC). We aimed to evaluate HDAC5 expression in human breast tumors and to determine the effects of the inhibition of HDAC5 expression in BC cells. EXPERIMENTAL DESIGN: HDAC5 expression was evaluated in BC patients and was correlated with clinical features and with patient prognosis. Functional experiments were performed using shRNA and the selective HDAC inhibitor LMK-235 for HDAC5 knockdown and inhibition in BC cells. The synergistic effects of LMK-235 with the proteasome inhibitor bortezomib were also examined. RESULTS: HDAC5 was extensively expressed in human BC tissues, and high HDAC5 expression was associated with an inferior prognosis. Knockdown of HDAC5 inhibited cell proliferation, migration, invasion, and enhanced apoptosis. The HDAC5 inhibitor LMK-235 inhibited cell growth and induced apoptosis, while the inclusion of bortezomib synergistically enhanced the efficacy of LMK-235. CONCLUSIONS: Our findings indicate that HDAC5 is a promising prognostic marker and drug target for BC and that the combination of LMK-235 and bortezomib presents a novel therapeutic strategy for BC.
Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells.[Pubmed:23252603]
J Med Chem. 2013 Jan 24;56(2):427-36.
The synthesis and biological evaluation of new potent hydroxamate-based HDAC inhibitors with a novel alkoxyamide connecting unit linker region are described. Biological evaluation includes MTT and cellular HDAC assays on sensitive and chemoresistant cancer cell lines as well as HDAC profiling of selected compounds. Compound 19i (LMK235) (N-((6-(hydroxyamino)-6-oxohexyl)oxy)-3,5-dimethylbenzamide) showed similar effects compared to vorinostat on inhibition of cellular HDACs in a pan-HDAC assay but enhanced cytotoxic effects against the human cancer cell lines A2780, Cal27, Kyse510, and MDA-MB231. Subsequent HDAC profiling yielded a novel HDAC isoform selectivity profile of 19i in comparison to vorinostat or trichostatin A (TSA). 19i shows nanomolar inhibition of HDAC4 and HDAC5, whereas vorinostat and TSA inhibit HDAC4 and HDAC5 in the higher micromolar range.


