Ginsenoside Rg3CAS# 14197-60-5 |
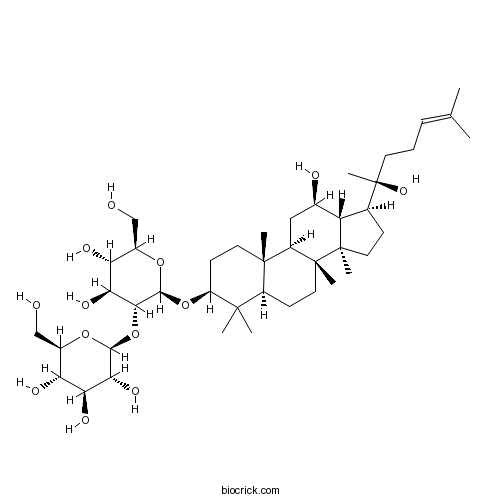
- 20(R)-Ginsenoside Rg3
Catalog No.:BCN5018
CAS No.:38243-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14197-60-5 | SDF | Download SDF |
| PubChem ID | 9918693 | Appearance | White powder |
| Formula | C42H72O13 | M.Wt | 785.02 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 20(S)-Ginsenoside-Rg3; Rg3; S-Ginsenoside Rg3;11019-45-7 | ||
| Solubility | DMSO : ≥ 50 mg/mL (63.69 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-2-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(C(O6)CO)O)O)O)C)C)O)C)O)C | ||
| Standard InChIKey | RWXIFXNRCLMQCD-JBVRGBGGSA-N | ||
| Standard InChI | InChI=1S/C42H72O13/c1-21(2)10-9-14-42(8,51)22-11-16-41(7)29(22)23(45)18-27-39(5)15-13-28(38(3,4)26(39)12-17-40(27,41)6)54-37-35(33(49)31(47)25(20-44)53-37)55-36-34(50)32(48)30(46)24(19-43)52-36/h10,22-37,43-51H,9,11-20H2,1-8H3/t22-,23+,24+,25+,26-,27+,28-,29-,30+,31+,32-,33-,34+,35+,36-,37-,39-,40+,41+,42-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ginsenoside Rg3 is the main component of Red ginseng, it inhibits Na+ and hKv1.4 channel with IC50s of 32.2±4.5 and 32.6±2.2 μM, respectively, it also inhibits Aβ levels, NF-κB activity, and COX-2 expression. Ginsenoside-Rg3 possesses an ability to inhibit the lung metastasis of tumor cells, and the mechanism of its antimetastatic effect is related to inhibition of the adhesion and invasion of tumor cells, and also to anti-angiogenesis activity. It is a novel drug, capable of inhibiting the early of scarring (HS) and later HS. |
| Targets | EGFR | NF-kB | IkB | Caspase | Bcl-2/Bax | Akt | NOS | c-Myc | p21 | IKK | Sodium Channel | hKv1.4 channel |
| In vitro | Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers.[Pubmed: 25744465]Biol Pharm Bull. 2015;38(1):102-8.Autophagy has been an emerging field in the treatment of hepatic carcinoma since anticancer therapies were shown to ignite autophagy in vitro and in vivo. Here we report that Ginsenoside Rg3 and Rh2, major components of red ginseng, induce apoptotic cell death in a stereoisomer-specific fashion.
Ginsenoside Rg3 sensitizes human non‑small cell lung cancer cells to γ-radiation by targeting the nuclear factor-κB pathway.[Pubmed: 25738799]Mol Med Rep. 2015 Jul;12(1):609-14.At present, it is elusive how non-small cell lung cancer (NSCLC) develops resistance to γ-radiation; however, the transcription factor nuclear factor-κB (NF-κB) and NF-κB-regulated gene products have been proposed as mediators. Ginsenoside Rg3 is a steroidal saponin, which was isolated from Panax ginseng. Ginsenoside Rg3 possesses high pharmacological activity and has previously been shown to suppress NF-κB activation in various types of tumor cell. Therefore, the present study aimed to determine whether Rg3 could suppress NF-κB activation in NSCLC cells and sensitize NSCLC to γ-radiation, using an NSCLC cell line and NSCLC xenograft.
|
| In vivo | Novel roles of ginsenoside Rg3 in apoptosis through downregulation of epidermal growth factor receptor.[Pubmed: 25824408]Chem Biol Interact. 2015 May 25;233:25-34.Ginsenoside Rg3 (Rg3), a pharmacologically active compound from red ginseng, has been reported to induce cell death in various cancer cell lines, although the specific mechanisms have not been well established. In the present study, Ginsenoside Rg3 treatment to A549 human lung adenocarcinoma led to cell death via not only apoptotic pathways but also the downregulation of epidermal growth factor receptor (EGFR).
In vivo inhibition of hypertrophic scars by implantable ginsenoside-Rg3-loaded electrospun fibrous membranes.[Pubmed: 23938200]Acta Biomater. 2013 Dec;9(12):9461-73.Clinically, hypertrophic scarring (HS) is a major concern for patients and has been a challenge for surgeons, as there is a lack of treatments that can intervene early in the formation of HS.
This study reports on a Chinese drug, 20(R)-Ginsenoside Rg3 (GS-Rg3), which can inhibit in vivo the early formation of HS and later HS hyperplasia by inducing the apoptosis of fibroblasts, inhibiting inflammation and down-regulating VEGF expression. |
| Cell Research | Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the B‑cell lymphoma/B‑cell lymphoma‑associated X protein pathway.[Pubmed: 25672441]Mol Med Rep. 2015 Jun;11(6):4518-24.Previous studies have suggested that Ginsenoside Rg3 (GSRg3) extract from the medicinal plant Panax ginseng, may increase nitric oxide production via increases in the phosphorylation and expression of endothelial nitric oxide synthase (eNOS).
|
| Animal Research | Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling.[Pubmed: 22101440 ]J Nat Med. 2012 Jul;66(3):476-85.Since prostate growth is governed by the androgen signaling pathway, blockade of the pathway is regarded as an appropriate strategy for the treatment of benign prostatic hyperplasia (BPH). Panax ginseng is known to have various pharmacological activities. Of several products of its root, red ginseng, having many bioactive ginsenosides, is most popularly used in Korea, and recently has been reported to control the proliferation of cancer cells. We here tested the effect of a water extract of Korean red ginseng (WKRG) on testosterone-induced prostate hyperplasia.
|

Ginsenoside Rg3 Dilution Calculator

Ginsenoside Rg3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2739 mL | 6.3693 mL | 12.7385 mL | 25.4771 mL | 31.8463 mL |
| 5 mM | 0.2548 mL | 1.2739 mL | 2.5477 mL | 5.0954 mL | 6.3693 mL |
| 10 mM | 0.1274 mL | 0.6369 mL | 1.2739 mL | 2.5477 mL | 3.1846 mL |
| 50 mM | 0.0255 mL | 0.1274 mL | 0.2548 mL | 0.5095 mL | 0.6369 mL |
| 100 mM | 0.0127 mL | 0.0637 mL | 0.1274 mL | 0.2548 mL | 0.3185 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ginsenoside Rg3 is the main component of Red ginseng. Ginsenoside Rg3 inhibits Na+ and hKv1.4 channel with IC50s of 32.2±4.5 and 32.6±2.2 μM, respectively. Ginsenoside Rg3 also inhibits Aβ levels, NF-κB activity, and COX-2 expression.
In Vitro:Ginsenoside Rg3 plays an important role in its effect on the Na+ channel. Treatment with Ginsenoside Rg3 reversibly inhibits the inward Na+ peak current (INa) with an IC50 of 32.2±4.5 μM, and the inhibition is voltage-dependent[1]. Ginsenoside Rg3 at 100 μM inhibits the hKv1.4 channel currents by an average of 65%.The Ginsenoside Rg3 effect is concentration-dependent and reversible. The IC50 value and Hill coefficient are 32.6±2.2 μM and 1.59±0.13, respectively[2]. Ginsenoside Rg3 shows the significant inhibition of NF-κB activity thereby reduced COX-2 expression. To examine the cytotoxicity of Ginsenoside Rg3 on IL-1β-induced inflamed A549 cells, the cells are firstly treated with IL-1β (10 ng/mL) for 4 h and treated with 100 to 900 ng/mL concentration of Ginsenoside Rg3 for 12 h. Cell viability is analyzed using an MTT assay. There is no observed cytotoxicity of Ginsenoside Rg3 in IL-1β-induced inflamed A549 cells compared to only PBS-treated cells (Con).To obtain the anti-inflammatory effects of Ginsenoside Rg3 on inflammation induced human lung epithelial cells, A549 cells inflammation is induced by IL-1β (10 ng/mL) and then treated by 5 μM of Dexamethasone (Dex) or 900 nM of Rg3. The NF-κB activation is analyzed by a western blot analysis to evaluate the effect of Ginsenoside Rg3 treatment on A549 cells. Phospho-NF-κB p65/total NF-κB p65 densitometry in the cells treated with Rg3 shows the significant decrease compared to IL-1β-induced inflamed A549 cells. The meaning of reducing the ratio of p-p65/p65 by Rg3 treatment is associated with NF-κB activation. Ginsenoside Rg3 also downregulates the expression of COX-2 effectively[3].
In Vivo:Ginsenoside Rg3 ((20S)-Rg3) is an Aβ-lowering Natural Compound. APP/PS1 mice are treated with Ginsenoside Rg3 once a day for 4 weeks by intraperitoneal injection (10 mg/kg/day). Aβ ELISA analysis of brain tissues reveal that Ginsenoside Rg3 treatment results in a significant reduction of Aβ40 and Aβ42 in the brain[4].
References:
[1]. Kim JH, et al. A role for the carbohydrate portion of ginsenoside Rg3 in Na+ channel inhibition. Mol Cells. 2005 Feb 28;19(1):137-42.
[2]. Lee JH, et al. Ginsenoside Rg3 inhibits human Kv1.4 channel currents by interacting with the Lys531 residue. Mol Pharmacol. 2008 Mar;73(3):619-26.
[3]. Lee IS, et al. Anti-Inflammatory Effects of Ginsenoside Rg3 via NF-κB Pathway in A549 Cells and Human Asthmatic Lung Tissue. J Immunol Res. 2016;2016:7521601.
[4]. Kang MS, et al. Modulation of lipid kinase PI4KIIα activity and lipid raft association of presenilin 1 underlies γ-secretase inhibition by ginsenoside (20S)-Rg3. J Biol Chem. 2013 Jul 19;288(29):20868-82.
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- 4-Hydroxyphenylacetonitrile
Catalog No.:BCN6905
CAS No.:14191-95-8
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- EI1
Catalog No.:BCC4044
CAS No.:1418308-27-6
- Minaxin C
Catalog No.:BCN7656
CAS No.:1418150-06-7
- LMK 235
Catalog No.:BCC2421
CAS No.:1418033-25-6
- NMS-873
Catalog No.:BCC4977
CAS No.:1418013-75-8
- Endomorphin-2
Catalog No.:BCC5697
CAS No.:141801-26-5
- Stauntosaponin A
Catalog No.:BCN6966
CAS No.:1417887-91-2
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
- Emetine Hydrochloride
Catalog No.:BCN2478
CAS No.:14198-59-5
- Methyl 4-Hydroxyphenylacetate
Catalog No.:BCN1571
CAS No.:14199-15-6
- NSC 625987
Catalog No.:BCC7269
CAS No.:141992-47-4
- NVP-TNKS656
Catalog No.:BCC6541
CAS No.:1419949-20-4
- Caproic acid
Catalog No.:BCC9218
CAS No.:142-62-1
- BIM 189
Catalog No.:BCC5934
CAS No.:142062-55-3
- Isosalvianolic acid C
Catalog No.:BCN3476
CAS No.:142115-17-1
- WS 3
Catalog No.:BCC7519
CAS No.:1421227-52-2
- WS6
Catalog No.:BCC5566
CAS No.:1421227-53-3
- (+)-Ketoconazole
Catalog No.:BCC4249
CAS No.:142128-59-4
In vivo inhibition of hypertrophic scars by implantable ginsenoside-Rg3-loaded electrospun fibrous membranes.[Pubmed:23938200]
Acta Biomater. 2013 Dec;9(12):9461-73.
Clinically, hypertrophic scarring (HS) is a major concern for patients and has been a challenge for surgeons, as there is a lack of treatments that can intervene early in the formation of HS. This study reports on a Chinese drug, 20(R)-Ginsenoside Rg3 (GS-Rg3), which can inhibit in vivo the early formation of HS and later HS hyperplasia by inducing the apoptosis of fibroblasts, inhibiting inflammation and down-regulating VEGF expression. Implantable biodegradable GS-Rg3-loaded poly(l-lactide) (PLA) fibrous membranes were successfully fabricated using co-electrospinning technology to control drug release and improve drug utilization. The in vivo releasing time of GS-Rg3 lasts for 3 months, and the drug concentration released in rabbits can be controlled by varying the drug content of the electrospun fibers. Histological observations of HE staining indicate that GS-Rg3/PLA significantly inhibits the HS formation, with obvious improvements in terms of dermis layer thickness, epidermis layer thickness and fibroblast proliferation. The results of immunohistochemistry staining and Masson's trichrome staining demonstrate that GS-Rg3/PLA electrospun fibrous membranes significantly inhibit HS formation, with decreased expression of collagen fibers and microvessels. VEGF protein levels are much lower in the group treated with GS-Rg3/PLA eletrospun membranes compared with other groups. These results demonstrate that GS-Rg3 is a novel drug, capable of inhibiting the early formation of HS and later HS hyperplasia. GS-Rg3/PLA electrospun membrane is a very promising new treatment for early and long-term treatment of HS.
Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the Bcell lymphoma/Bcell lymphomaassociated X protein pathway.[Pubmed:25672441]
Mol Med Rep. 2015 Jun;11(6):4518-24.
Previous studies have suggested that Ginsenoside Rg3 (GSRg3) extract from the medicinal plant Panax ginseng, may increase nitric oxide production via increases in the phosphorylation and expression of endothelial nitric oxide synthase (eNOS). The present study used an in vitro neonatal rat cardiomyocyte (NRC) model of anoxiareoxygenation injury and an in vivo rat model of myocardial ischemia/reperfusion (MI/R) injury. Hemodynamic, histopathological and biochemical assessment of the myocardial injury was performed and the expression levels of lactate dehydrogenase (LDH), superoxide dismutase and creatine kinase (CK) were measured in serum from the animal model, which may reflect myocardial injury. NRC injury was determined using a Cell Counting kit8. The GSRg3 antiapoptotic effects were assessed using flow cytometry to investigate the number of earlylate apoptotic cells and western blot analysis was performed to analyze the protein expression levels of caspase3, caspase9, Bcell lymphoma2 (Bcl2), phosphorylated (p)Akt and eNOS. The results suggested that pretreatment with GSRg3 (60 mg/kg) significantly improved rat cardiac function, as demonstrated by increased left ventricular systolic pressure, heart rate and first derivative of left ventricular pressure. GSRg3 also reduced the size of the myocardial infarct and LDH/CK levels in the blood following MI/R. In vitro investigations revealed that GSRg3 (10 mM) decreased NRC apoptosis through inhibiting the activation of caspase3 and caspase9, and increasing the expression levels of pAkt, eNOS and the ratio of Bcl2/Bcl2associated X protein (Bax). Overall, the present study revealed that GSRg3 mediated a cardioprotective effect against MI/Rinduced apoptosis via Akt/eNOS signaling and the Bcl2/Bax pathway.
Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling.[Pubmed:22101440]
J Nat Med. 2012 Jul;66(3):476-85.
Since prostate growth is governed by the androgen signaling pathway, blockade of the pathway is regarded as an appropriate strategy for the treatment of benign prostatic hyperplasia (BPH). Panax ginseng is known to have various pharmacological activities. Of several products of its root, red ginseng, having many bioactive ginsenosides, is most popularly used in Korea, and recently has been reported to control the proliferation of cancer cells. We here tested the effect of a water extract of Korean red ginseng (WKRG) on testosterone-induced prostate hyperplasia. WKRG (daily intraperitoneal injection) prevented prostate overgrowth and epithelial thickening induced by testosterone in rats, and suppressed a rat prostate kallikrein-S3. In human prostate cells, WKRG inhibited testosterone-induced cell proliferation, arrested cell cycle by inducing p21 and p27, and induced apoptosis. Testosterone-induced expression of human kallikrein-3 mRNA and activation of androgen receptor (AR) were effectively inhibited by WKRG. Of the major ginsenosides included in WKRG, 20(S)-Rg3 was identified to repress AR activity and to attenuate prostate cell growth during testosterone stimulation. Moreover, 20(S)-Rg3 downregulated AR by facilitating the degradation of AR protein. WKRG and 20(S)-Rg3 were found to have new pharmacological activities against testosterone-induced prostate overgrowth. Given that red ginseng has been used safely in Asia for 1000 years, red ginseng and 20(S)-Rg3 could be potential therapeutic regimens for treating BPH.
Ginsenoside Rg3 sensitizes human non-small cell lung cancer cells to gamma-radiation by targeting the nuclear factor-kappaB pathway.[Pubmed:25738799]
Mol Med Rep. 2015 Jul;12(1):609-14.
At present, it is elusive how non-small cell lung cancer (NSCLC) develops resistance to gamma-radiation; however, the transcription factor nuclear factor-kappaB (NF-kappaB) and NF-kappaB-regulated gene products have been proposed as mediators. Ginsenoside Rg3 is a steroidal saponin, which was isolated from Panax ginseng. Ginsenoside Rg3 possesses high pharmacological activity and has previously been shown to suppress NF-kappaB activation in various types of tumor cell. Therefore, the present study aimed to determine whether Rg3 could suppress NF-kappaB activation in NSCLC cells and sensitize NSCLC to gamma-radiation, using an NSCLC cell line and NSCLC xenograft. A clone formation assay and lung tumor xenograft experiment were used to assess the radiosensitizing effects of Ginsenoside Rg3. NF-kappaB/inhibitor of NF-kappaB (IkappaB) modulation was ascertained using an electrophoretic mobility shift assay and western blot analysis. NF-kappaB-regulated gene products were monitored by western blot analysis. The present study demonstrated that Ginsenoside Rg3 was able to sensitize A549 and H1299 lung carcinoma cells to gamma-radiation and significantly enhance the efficacy of radiation therapy in C57BL/6 mice bearing a Lewis lung carcinoma cell xenograft tumor. Furthermore, Ginsenoside Rg3 suppressed NF-kappaB activation, phosphorylation of IkappaB protein and expression of NF-kappaB-regulated gene products (cyclin D1, c-myc, B-cell lymphoma 2, cyclooxygenase-2, matrix metalloproteinase-9 and vascular endothelial growth factor), a number of which were induced by radiation therapy and mediate radioresistance. In conclusion, the results of the present study suggested that Ginsenoside Rg3 may potentiate the antitumor effects of radiation therapy in NSCLC by suppressing NF-kappaB activity and NF-kappaB-regulated gene products, leading to the inhibition of tumor progression.
Novel roles of ginsenoside Rg3 in apoptosis through downregulation of epidermal growth factor receptor.[Pubmed:25824408]
Chem Biol Interact. 2015 May 25;233:25-34.
Ginsenoside Rg3 (Rg3), a pharmacologically active compound from red ginseng, has been reported to induce cell death in various cancer cell lines, although the specific mechanisms have not been well established. In the present study, Rg3 treatment to A549 human lung adenocarcinoma led to cell death via not only apoptotic pathways but also the downregulation of epidermal growth factor receptor (EGFR). We used cross-linker and cell enzyme-linked immunosorbent assays to show that Rg3 inhibited EGFR dimerization by EGF stimulation and caused EGFR internalization from the cell membrane. Among several important phosphorylation sites in cytoplasmic EGFR, Rg3 increased the phosphorylation of tyrosine 1045 (pY1045) and serine 1046/1047 (pS1046/1047) for EGFR degradation and coincidently, attenuated pY1173 and pY1068 for mitogen-activated protein kinase activity. These effects were amplified under EGF-pretreated Rg3 stimulation. In vivo experiments showed that the average volume of the tumors treated with 30 mg/kg of Rg3 was significantly decreased by 40% compared with the control. Through immunohistochemistry, we detected the fragmentation of DNA, the accumulation of Rg3, and the reduction of EGFR expression in the Rg3-treated groups. Here, we provide the first description of the roles of Rg3 in the reduction of cell surface EGFR, the attenuation of EGFR signal transduction, and the eventual activation of apoptosis in A549 human lung adenocarcinoma.
Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers.[Pubmed:25744465]
Biol Pharm Bull. 2015;38(1):102-8.
Autophagy has been an emerging field in the treatment of hepatic carcinoma since anticancer therapies were shown to ignite autophagy in vitro and in vivo. Here we report that Ginsenoside Rg3 and Rh2, major components of red ginseng, induce apoptotic cell death in a stereoisomer-specific fashion. The 20(S)-forms of Rg3 and Rh2, but not their respective 20(R)-forms, promoted cell death in a dose-dependent manner accompanied by downregulation of Bcl2 and upregulation of Fas, resulting in apoptosis of HepG2 cells with poly ADP ribose polymerase cleavage. The LD50 value [45 microM for Rg3(S), less than 10 microM for Rh2(S)] and gross morphological electron microscopic observation revealed more severe cellular damage in cells treated with Rh2(S) than in those treated with Rg3(S). Both Rg3(S) and Rh2(S) also induced autophagy when undergoing induced apoptosis. Inhibition of autophagy with lysosomotrophic agents significantly potentiated the cellular damage, implying a favorable switch of the cell fate to tumor cell death. Blocking intracellular calcium with 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) restored the cell death induced by both Rg3(S) and Rh2(S). Our results suggest that the 20(S)-forms of Rg3 and Rh2 in red ginseng possess more potent antitumor activity with autophagy than their 20(R)-forms via calcium-dependent apoptosis.


