BIM 189Bombesin antagonist CAS# 142062-55-3 |
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 142062-55-3 | SDF | Download SDF |
| PubChem ID | 5748499 | Appearance | Powder |
| Formula | C56H73ClN14O10 | M.Wt | 1137.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | [D-Phe<sup>6</sup>,Leu<sup>13</sup>,Cpa<sup>14</sup>]bombesin-(6-14)NH<sub>2</sub> | ||
| Solubility | Soluble to 2 mg/ml in 20% acetonitrile | ||
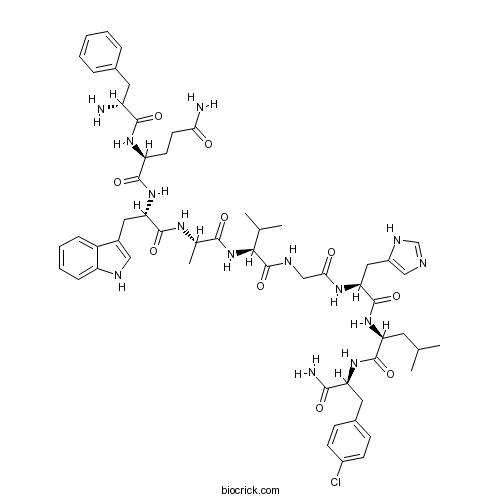
| Sequence | FQWAVGHLF (Modifications: Phe-1 = D-Phe, Phe-9 = 4-Cl-Phe & C-terminal amide) | ||
| Chemical Name | (2S)-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[[(2S)-1-[[(2S)-1-amino-3-(4-chlorophenyl)-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]-2-[[(2R)-2-amino-3-phenylpropanoyl]amino]pentanediamide | ||
| SMILES | CC(C)CC(C(=O)NC(CC1=CC=C(C=C1)Cl)C(=O)N)NC(=O)C(CC2=CN=CN2)NC(=O)CNC(=O)C(C(C)C)NC(=O)C(C)NC(=O)C(CC3=CNC4=CC=CC=C43)NC(=O)C(CCC(=O)N)NC(=O)C(CC5=CC=CC=C5)N | ||
| Standard InChIKey | XJWMPWLZAVKSPK-UYBYNCNQSA-N | ||
| Standard InChI | InChI=1S/C56H73ClN14O10/c1-30(2)21-43(54(79)68-42(49(60)74)23-34-15-17-36(57)18-16-34)69-55(80)45(25-37-27-61-29-64-37)66-47(73)28-63-56(81)48(31(3)4)71-50(75)32(5)65-53(78)44(24-35-26-62-40-14-10-9-13-38(35)40)70-52(77)41(19-20-46(59)72)67-51(76)39(58)22-33-11-7-6-8-12-33/h6-18,26-27,29-32,39,41-45,48,62H,19-25,28,58H2,1-5H3,(H2,59,72)(H2,60,74)(H,61,64)(H,63,81)(H,65,78)(H,66,73)(H,67,76)(H,68,79)(H,69,80)(H,70,77)(H,71,75)/t32-,39+,41-,42-,43-,44-,45-,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bombesin antagonist that reduces bombesin-induced satiety. |

BIM 189 Dilution Calculator

BIM 189 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 10.4 nM for rat pancreas; 2.4 nM for Guinea pig pancreas
Bombesin has common effects on the gastrointestinal tract and feeding behavior. Bombesin acts on two types of receptors: one with high affinity for neuromedin B and another with high affinity for bombesin and gastrin-releasing peptide (GRP). BIM 189 is a a new peptide of bombesin antagonis, while BIM 187 is a bombesin agonist.
In vitro: BIM 189 was one of the most potent bombesin antagonists known in the guinea pig and 3T3 cell systems but has 40% partial agonist activity in the rat. Loss of agonism might be attributed to the Cl electron withdrawing effects rather than the size of the Cl since far larger Na114 substitutions did not effect agonist activity [2].
In vivo: BIM 187 at 4 μg/kg, significantly reduced food intake at 30 min in rat, but did not change the total 6-h food intake. BIM 189 (10 mg/kg), had no effect on food intake when administered alone, even at high doses (20 mg/kg). BIM 189 selectively reduced bombesin-induced satiety but had no effect on satiety induced by BIM 187[2].
Clinical trial: Up to now, BIM 189 is still in the preclinical development stage.
Reference:
[1] Coy D, Wang LH, Jiang NY, Jensen R. Short chain bombesin pseudopeptides with potent bombesin receptor antagonist activity in rat and guinea pig pancreatic acinar cells. Eur J Pharmacol. 1990 Nov 6;190(1-2):31-8.
[2] Laferrère B, Leroy F, Bonhomme G, Le Gall A, Basdevant A, Guy-Grand B. Effects of bombesin, of a new bombesin agonist (BIM 187) and a new antagonist (BIM 189) on food intake in rats, in relation to cholecystokinin. Eur J Pharmacol. 1992 Apr 29;215(1):23-8.
- Caproic acid
Catalog No.:BCC9218
CAS No.:142-62-1
- NVP-TNKS656
Catalog No.:BCC6541
CAS No.:1419949-20-4
- NSC 625987
Catalog No.:BCC7269
CAS No.:141992-47-4
- Methyl 4-Hydroxyphenylacetate
Catalog No.:BCN1571
CAS No.:14199-15-6
- Emetine Hydrochloride
Catalog No.:BCN2478
CAS No.:14198-59-5
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
- Ginsenoside Rg3
Catalog No.:BCN1068
CAS No.:14197-60-5
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- 4-Hydroxyphenylacetonitrile
Catalog No.:BCN6905
CAS No.:14191-95-8
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- Isosalvianolic acid C
Catalog No.:BCN3476
CAS No.:142115-17-1
- WS 3
Catalog No.:BCC7519
CAS No.:1421227-52-2
- WS6
Catalog No.:BCC5566
CAS No.:1421227-53-3
- (+)-Ketoconazole
Catalog No.:BCC4249
CAS No.:142128-59-4
- CYM 50769
Catalog No.:BCC6337
CAS No.:1421365-63-0
- Mutated EGFR-IN-1
Catalog No.:BCC5444
CAS No.:1421372-66-8
- Mutant EGFR inhibitor
Catalog No.:BCC4119
CAS No.:1421373-62-7
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- AZD-9291 mesylate
Catalog No.:BCC4121
CAS No.:1421373-66-1
- AZ5104
Catalog No.:BCC6389
CAS No.:1421373-98-9
- LY3039478
Catalog No.:BCC2105
CAS No.:1421438-81-4
- Sweroside
Catalog No.:BCN6219
CAS No.:14215-86-2
Effects of bombesin, of a new bombesin agonist (BIM187) and a new antagonist (BIM189) on food intake in rats, in relation to cholecystokinin.[Pubmed:1516647]
Eur J Pharmacol. 1992 Apr 29;215(1):23-8.
To study the mechanism by which bombesin induces satiety, we studied the effect of two new peptides, BIM187, a bombesin agonist, and BIM189, a bombesin antagonist, on food intake in rats fed 6 h a day. BIM187 at 4 micrograms/kg, significantly reduced food intake at 30 min, but did not change the total 6-h food intake. BIM189 (10 mg/kg), had no effect on food intake when administered alone, even at high doses (20 mg/kg). BIM189 selectively reduced bombesin-induced satiety but had no effect on satiety induced by BIM187. To examine the extent to which the satiety effect of bombesin or related peptides depends on the release of cholecystokinin (CCK), we studied the ability of CCK antagonists, BIM18216 and L364718, to reduce satiety induced by bombesin and BIM187. Neither BIM18216 nor L364718 alone had an effect on the 30-min food intake. They were not able to reverse the effect of bombesin on food intake. In our model, bombesin seems to act on satiety by a mechanism independent of CCK.
Short chain bombesin pseudopeptides with potent bombesin receptor antagonist activity in rat and guinea pig pancreatic acinar cells.[Pubmed:1963850]
Eur J Pharmacol. 1990 Nov 6;190(1-2):31-8.
A series of potent bombesin antagonists based on the reduced C-terminal peptide bond modification which in the past resulted in the first really potent antagonists are compared for effects on bombesin-stimulated amylase release from and binding to rat and guinea pig pancreatic acini. It was found that the original member of this series, [Leu13 psi (CH2NH)Leu14] bombesin, displayed partial agonist activity with 11% efficacy in the rat. More recent analogues of this type which were found previously to be even more potent pure antagonists in the guinea pig pancreas or 3T3 cells, exhibited similarly higher binding affinity for rat acini but displayed even higher residual partial agonist activity in the rat. For instance, [D-Phe6,Leu13 psi (CH2NH)Phe14]bombesin-(6-14) was one of the most potent bombesin antagonists known in the guinea pig and 3T3 cell systems but has 40% partial agonist activity in the rat. Several structural modification strategies were developed to remove rat partial agonist properties with retention of high antagonist potency in all systems tested. The most effective of these was the substitution of a Cl on the aromatic ring of the Phe residue (p-Cl-Phe, Cpa) in position 14 to give [D-Phe6,Leu13 psi (CH2NH)Cpa14]bombesin-(6-14). This had higher binding affinities for both rat and guinea pig pancreatic acini and was a pure antagonist on both cell types. Another effective method was alteration of the stereochemistry of the position 14 amino acid in [D-Phe6,Leu13 psi (CH2ND)D-Phe14]bombesin-(6-14) which had somewhat lowered binding affinities but pure antagonist properties.(ABSTRACT TRUNCATED AT 250 WORDS)


