Mutated EGFR-IN-1CAS# 1421372-66-8 |
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Viomycin
Catalog No.:BCC3930
CAS No.:32988-50-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1421372-66-8 | SDF | Download SDF |
| PubChem ID | 78358313 | Appearance | Powder |
| Formula | C25H31N7O | M.Wt | 445.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 75 mg/mL (168.33 mM) *"≥" means soluble, but saturation unknown. | ||
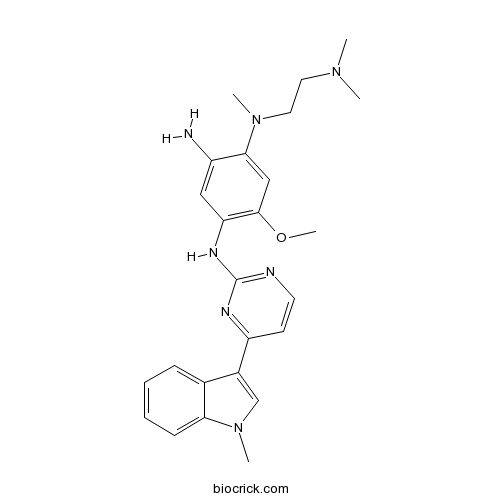
| Chemical Name | 1-N-[2-(dimethylamino)ethyl]-5-methoxy-1-N-methyl-4-N-[4-(1-methylindol-3-yl)pyrimidin-2-yl]benzene-1,2,4-triamine | ||
| SMILES | CN1C=C(C2=CC=CC=C21)C3=NC(=NC=C3)NC4=C(C=C(C(=C4)N)N(C)CCN(C)C)OC | ||
| Standard InChIKey | HTNTZPBKKCORTP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H31N7O/c1-30(2)12-13-31(3)23-15-24(33-5)21(14-19(23)26)29-25-27-11-10-20(28-25)18-16-32(4)22-9-7-6-8-17(18)22/h6-11,14-16H,12-13,26H2,1-5H3,(H,27,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mutated EGFR-IN-1 Dilution Calculator

Mutated EGFR-IN-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2444 mL | 11.2218 mL | 22.4437 mL | 44.8873 mL | 56.1092 mL |
| 5 mM | 0.4489 mL | 2.2444 mL | 4.4887 mL | 8.9775 mL | 11.2218 mL |
| 10 mM | 0.2244 mL | 1.1222 mL | 2.2444 mL | 4.4887 mL | 5.6109 mL |
| 50 mM | 0.0449 mL | 0.2244 mL | 0.4489 mL | 0.8977 mL | 1.1222 mL |
| 100 mM | 0.0224 mL | 0.1122 mL | 0.2244 mL | 0.4489 mL | 0.5611 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mutated EGFR-IN-1 is a useful intermediate for the inhibitors design for mutated EGFR, such as L858R EGFR, Exonl9 deletion activating mutant and T790M resistance mutant. IC50 value: Target: Mutated EGFR inhibitor More information can be found in Patent WO 2013014448 A1.2 - (2, 4, 5 - substituted -anilino) pyrimidine derivatives as egfr modulators useful for treating cancer.
References:
[1]. Patent WO 2013014448 A1. 2 - (2, 4, 5 - substituted -anilino) pyrimidine derivatives as egfr modulators useful for treating cancer
.
- CYM 50769
Catalog No.:BCC6337
CAS No.:1421365-63-0
- (+)-Ketoconazole
Catalog No.:BCC4249
CAS No.:142128-59-4
- WS6
Catalog No.:BCC5566
CAS No.:1421227-53-3
- WS 3
Catalog No.:BCC7519
CAS No.:1421227-52-2
- Isosalvianolic acid C
Catalog No.:BCN3476
CAS No.:142115-17-1
- BIM 189
Catalog No.:BCC5934
CAS No.:142062-55-3
- Caproic acid
Catalog No.:BCC9218
CAS No.:142-62-1
- NVP-TNKS656
Catalog No.:BCC6541
CAS No.:1419949-20-4
- NSC 625987
Catalog No.:BCC7269
CAS No.:141992-47-4
- Methyl 4-Hydroxyphenylacetate
Catalog No.:BCN1571
CAS No.:14199-15-6
- Emetine Hydrochloride
Catalog No.:BCN2478
CAS No.:14198-59-5
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
- Mutant EGFR inhibitor
Catalog No.:BCC4119
CAS No.:1421373-62-7
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- AZD-9291 mesylate
Catalog No.:BCC4121
CAS No.:1421373-66-1
- AZ5104
Catalog No.:BCC6389
CAS No.:1421373-98-9
- LY3039478
Catalog No.:BCC2105
CAS No.:1421438-81-4
- Sweroside
Catalog No.:BCN6219
CAS No.:14215-86-2
- Hederacoside C
Catalog No.:BCN2329
CAS No.:14216-03-6
- GZD824
Catalog No.:BCC4389
CAS No.:1421783-64-3
- KPT-276
Catalog No.:BCC4445
CAS No.:1421919-75-6
- Taxumairol B
Catalog No.:BCN6940
CAS No.:142203-64-3
- 9-Dihydro-13-acetylbaccatin III
Catalog No.:BCC1315
CAS No.:142203-65-4
- Catestatin
Catalog No.:BCC5935
CAS No.:142211-96-9
Discordant Genotypic Sex and Phenotype Variations in Two Spanish Siblings with 17alpha-Hydroxylase/17,20-Lyase Deficiency Carrying the Most Prevalent Mutated CYP17A1 Alleles of Brazilian Patients.[Pubmed:28376482]
Sex Dev. 2017;11(2):70-77.
17alpha-hydroxylase/17,20-lyase deficiency is a rare form of congenital adrenal hyperplasia caused by mutations in CYP17A1. Two phenotypic female sisters, aged 17 and 15 years and with 46,XY and 46,XX karyotypes, respectively, presented with primary amenorrhea and absent secondary sexual characteristics. The elder sib also presented with high blood pressure. Both patients had elevated levels of ACTH, gonadotropins, progesterone, corticosterone, and deoxycorticosterone, and reduced levels of estradiol, testosterone, androstenedione, 17-OH-P, DHEA-S, cortisol, aldosterone, and renin activity. The CYP17A1 gene was sequenced, and polymorphic haplotypes were further analyzed in the Spanish family and in Brazilian patients. The 2 sisters were compound heterozygous for p.Arg362Cys and p.Trp406Arg mutations, previously described as the most prevalent mutations in Brazilian families of Spanish (p.Trp406Arg) or Portuguese (p.Arg362Cys) origin. Analysis of polymorphisms in CYP17A1 suggested that the paternal allele with p.Arg362Cys may share a common origin with the Brazilian carriers, while the maternal allele with p.Trp406Arg did not. Hydrocortisone and sex hormone replacement therapy was initiated in both patients. In conclusion, one CYP17A1 mutation (p.Arg362Cys) may share a common ancestry in Brazilian and our present Spanish patients, while p.Trp406Arg may have arisen separately. The elder patient (46,XY) developed a more severe phenotype and a poorer response to estradiol replacement therapy.
MET amplification and epithelial-to-mesenchymal transition exist as parallel resistance mechanisms in erlotinib-resistant, EGFR-mutated, NSCLC HCC827 cells.[Pubmed:28368392]
Oncogenesis. 2017 Apr 3;6(4):e307.
Although many epidermal growth factor receptor (EGFR)-mutated lung cancer patients initially benefit from the EGFR-inhibitor erlotinib, all acquire resistance. So far, several mechanisms implicated in resistance have been identified, but the existence of multiple resistance mechanisms in parallel have only been sparsely investigated. In this study, we investigated parallel resistance mechanisms acquired by HCC827, an EGFR-mutated adenocarcinoma cell line dependent on EGFR activity and sensitive to erlotinib. The cell line was treated with erlotinib by stepwise escalation of the drug-concentration and erlotinib-resistant (HCC827ER) cells created. HCC827ER cells depicted a mixed epithelial and mesenchymal phenotype. To clarify potential parallel resistance mechanisms, 14 resistant subclones were established by limited dilution. Interestingly, all HCC827ER subclones harbored either a MET-amplification (6/14) or underwent EMT (8/14), mechanisms both found in previous studies, but not in co-occurrence. Both subclone-types were resistant to erlotinib, but only MET-subclones responded to the MET-inhibitors crizotinib and capmatinib. EMT-subclones on the other hand had markedly increased FGFR1 expression and responded to the FGFR-inhibitor AZD4547, whereas MET-subclones did not. Monitoring gene expression through the development of HCC827ER revealed upregulation of FGFR1 expression as an early response to erlotinib. In addition, FGFR1 expression increased upon short-term erlotinib treatment (48 h) identifying a physiological role immediately after erlotinib exposure. The high FGFR1 expression seen in EMT-subclones was stable even after five passages without erlotinib. Here we show, that parallel resistance mechanisms appear during erlotinib-resistance development in EGFR-mutated NSCLC cells and highlight a role for FGFR1 expression changes as an early response to erlotinib as well as a bypass-signaling mechanism.


