GZD824Bcr-Abl inhibitor,novel orally bioavailable CAS# 1421783-64-3 |
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- GNF 5
Catalog No.:BCC3892
CAS No.:778277-15-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1421783-64-3 | SDF | Download SDF |
| PubChem ID | 71519689 | Appearance | Powder |
| Formula | C31H35F3N6O7S2 | M.Wt | 724.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
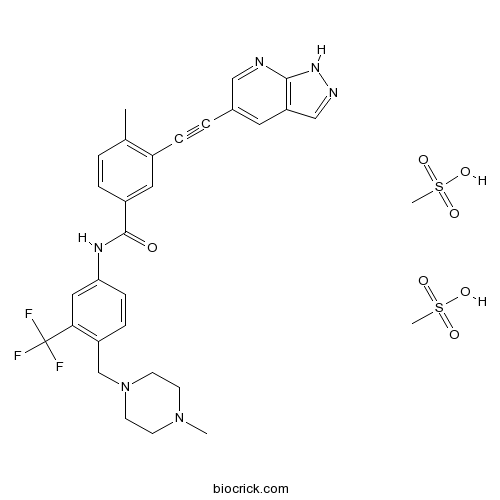
| Chemical Name | methanesulfonic acid;4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]-3-[2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl]benzamide | ||
| SMILES | CC1=C(C=C(C=C1)C(=O)NC2=CC(=C(C=C2)CN3CCN(CC3)C)C(F)(F)F)C#CC4=CC5=C(NN=C5)N=C4.CS(=O)(=O)O.CS(=O)(=O)O | ||
| Standard InChIKey | LEVIGHXVOVROGW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H27F3N6O.2CH4O3S/c1-19-3-5-22(14-21(19)6-4-20-13-24-17-34-36-27(24)33-16-20)28(39)35-25-8-7-23(26(15-25)29(30,31)32)18-38-11-9-37(2)10-12-38;2*1-5(2,3)4/h3,5,7-8,13-17H,9-12,18H2,1-2H3,(H,35,39)(H,33,34,36);2*1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

GZD824 Dilution Calculator

GZD824 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3797 mL | 6.8987 mL | 13.7975 mL | 27.595 mL | 34.4937 mL |
| 5 mM | 0.2759 mL | 1.3797 mL | 2.7595 mL | 5.519 mL | 6.8987 mL |
| 10 mM | 0.138 mL | 0.6899 mL | 1.3797 mL | 2.7595 mL | 3.4494 mL |
| 50 mM | 0.0276 mL | 0.138 mL | 0.2759 mL | 0.5519 mL | 0.6899 mL |
| 100 mM | 0.0138 mL | 0.069 mL | 0.138 mL | 0.2759 mL | 0.3449 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GZD824 is an orally bioavailable inhibitor of Bcr-Abl with IC50 values of 0.34 and 0.68 nM for Bcr-AblWT and Bcr-AblT315I, respectively [1].
Bcr-Abl is a fused protein that interacts with the interleukin-3 receptorβ(c) subunit and has tyrosine kinase activity. Abl activates cell cycle-related proteins and enzymes and increases cell division. Bcr-Abl inhibits DNA repair and causes genomic instability.
GZD824 is an orally bioavailable Bcr-Abl inhibitor. GZD824 exhibited high affinity with Kd values of 0.32 and 0.71 nM for Bcr-AblWT and Bcr-AblT315I, respectively. GZD824 inhibited Bcr-Abl with IC50 values of 0.34, 0.68, 0.27, 0.71, 0.15, 0.35, 0.29 and 0.35 nM for Bcr-AblWT, Bcr-AblT315I, Bcr-AblE255K, Bcr-AblG250E, Bcr-AblQ252H, Bcr-AblH396P, Bcr-AblM351T and Bcr-AblY253F, respectively. In a competitive binding assay, GZD824 bound to the ATP-binding sites of native Abl with Kd values of 0.32 and 0.34 nM for non-phosphorylated and phosphorylated Abl. In stably transformed Ba/F3 cells, GZD824 potently inhibited cells growth with IC50 values of 1.0 and 7.1 nM for Bcr-AblWT and Bcr-AblT315I expressed cells, respectively [1].
In mouse xenograft tumor models, GZD824 inhibited tumor growth. In mice bearing an allograft leukemia model, GZD824 significantly increased survival [1].
Reference:
Ren X, Pan X, Zhang Z, et al. Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem, 2013, 56(3): 879-894.
- Hederacoside C
Catalog No.:BCN2329
CAS No.:14216-03-6
- Sweroside
Catalog No.:BCN6219
CAS No.:14215-86-2
- LY3039478
Catalog No.:BCC2105
CAS No.:1421438-81-4
- AZ5104
Catalog No.:BCC6389
CAS No.:1421373-98-9
- AZD-9291 mesylate
Catalog No.:BCC4121
CAS No.:1421373-66-1
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- Mutant EGFR inhibitor
Catalog No.:BCC4119
CAS No.:1421373-62-7
- Mutated EGFR-IN-1
Catalog No.:BCC5444
CAS No.:1421372-66-8
- CYM 50769
Catalog No.:BCC6337
CAS No.:1421365-63-0
- (+)-Ketoconazole
Catalog No.:BCC4249
CAS No.:142128-59-4
- WS6
Catalog No.:BCC5566
CAS No.:1421227-53-3
- WS 3
Catalog No.:BCC7519
CAS No.:1421227-52-2
- KPT-276
Catalog No.:BCC4445
CAS No.:1421919-75-6
- Taxumairol B
Catalog No.:BCN6940
CAS No.:142203-64-3
- 9-Dihydro-13-acetylbaccatin III
Catalog No.:BCC1315
CAS No.:142203-65-4
- Catestatin
Catalog No.:BCC5935
CAS No.:142211-96-9
- Protionamide
Catalog No.:BCC4834
CAS No.:14222-60-7
- 2'-Rhamnoechinacoside
Catalog No.:BCN8219
CAS No.:1422390-59-7
- Rauvotetraphylline A
Catalog No.:BCN7052
CAS No.:1422506-49-7
- Rauvotetraphylline B
Catalog No.:BCN7056
CAS No.:1422506-50-0
- Rauvotetraphylline C
Catalog No.:BCN7055
CAS No.:1422506-51-1
- Rauvotetraphylline D
Catalog No.:BCN7054
CAS No.:1422506-52-2
- Rauvotetraphylline E
Catalog No.:BCN7051
CAS No.:1422506-53-3
- Kenpaullone
Catalog No.:BCC7047
CAS No.:142273-20-9
Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib.[Pubmed:23301703]
J Med Chem. 2013 Feb 14;56(3):879-94.
Bcr-Abl(T315I) mutation-induced imatinib resistance remains a major challenge for clinical management of chronic myelogenous leukemia (CML). Herein, we report GZD824 (10a) as a novel orally bioavailable inhibitor against a broad spectrum of Bcr-Abl mutants including T315I. It tightly bound to Bcr-Abl(WT) and Bcr-Abl(T315I) with K(d) values of 0.32 and 0.71 nM, respectively, and strongly inhibited the kinase functions with nanomolar IC(50) values. The compound potently suppressed proliferation of Bcr-Abl-positive K562 and Ku812 human CML cells with IC(50) values of 0.2 and 0.13 nM, respectively. It also displayed good oral bioavailability (48.7%), a reasonable half-life (10.6 h), and promising in vivo antitumor efficacy. It induced tumor regression in mouse xenograft tumor models driven by Bcr-Abl(WT) or the mutants and significantly improved the survival of mice bearing an allograft leukemia model with Ba/F3 cells harboring Bcr-Abl(T315I). GZD824 represents a promising lead candidate for development of Bcr-Abl inhibitors to overcome acquired imatinib resistance.


