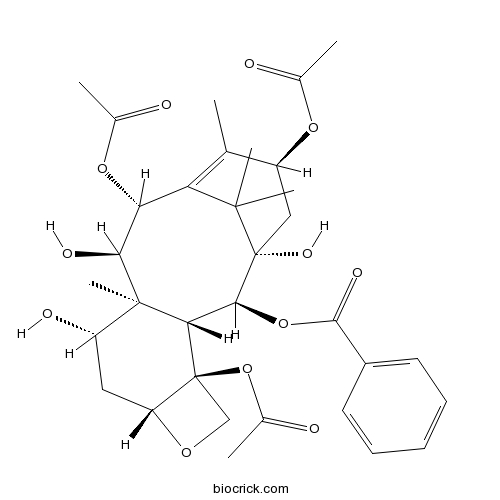
9-Dihydro-13-acetylbaccatin IIIAn intermediate for taxol analog preparations CAS# 142203-65-4 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
- Levistilide A
Catalog No.:BCN1197
CAS No.:88182-33-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 142203-65-4 | SDF | Download SDF |
| PubChem ID | 3083352 | Appearance | Powder |
| Formula | C33H42O12 | M.Wt | 630.68 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 9-DHAB III; 13-Acetyl-9-dihydrobaccatin III | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1=C2C(C(C3(C(CC4C(C3C(C(C2(C)C)(CC1OC(=O)C)O)OC(=O)C5=CC=CC=C5)(CO4)OC(=O)C)O)C)O)OC(=O)C | ||
| Standard InChIKey | WPPPFZJNKLMYBW-FAEUQDRCSA-N | ||
| Standard InChI | InChI=1S/C33H42O12/c1-16-21(42-17(2)34)14-33(40)28(44-29(39)20-11-9-8-10-12-20)26-31(7,22(37)13-23-32(26,15-41-23)45-19(4)36)27(38)25(43-18(3)35)24(16)30(33,5)6/h8-12,21-23,25-28,37-38,40H,13-15H2,1-7H3/t21-,22-,23+,25+,26-,27-,28-,31+,32-,33+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 9-Dihydro-13-acetylbaccatin III shows cytotoxicity against the MCF7 cell line and drug resistant cell line MCF7-ADR. |
| In vitro | Semi-synthesis of an O-glycosylated docetaxel analogue.[Pubmed: 12628678]Bioorg Med Chem. 2003 Apr 3;11(7):1551-6.A 7beta-O-glycosylated docetaxel analogue was semi-synthesized from 9-Dihydro-13-acetylbaccatin III, the most abundant taxane isolated from the needles of Taxus canadensis. |
| Structure Identification | Bioorg Med Chem. 2000 Jun;8(6):1269-80.Taxus canadensis abundant taxane: conversion to paclitaxel and rearrangements.[Pubmed: 10896107]An efficient conversion of Taxus canadensis abundant taxane, 9-Dihydro-13-acetylbaccatin III to baccatin III is described. Since the synthesis of paclitaxel from baccatin III has been reported, this work can be used for additional supply of this powerful anticancer drug. In addition, new taxanes derived from skeletal rearrangements originating from oxidation reduction reactions of the Canadian yew major taxane, are reported. |

9-Dihydro-13-acetylbaccatin III Dilution Calculator

9-Dihydro-13-acetylbaccatin III Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5856 mL | 7.928 mL | 15.8559 mL | 31.7118 mL | 39.6398 mL |
| 5 mM | 0.3171 mL | 1.5856 mL | 3.1712 mL | 6.3424 mL | 7.928 mL |
| 10 mM | 0.1586 mL | 0.7928 mL | 1.5856 mL | 3.1712 mL | 3.964 mL |
| 50 mM | 0.0317 mL | 0.1586 mL | 0.3171 mL | 0.6342 mL | 0.7928 mL |
| 100 mM | 0.0159 mL | 0.0793 mL | 0.1586 mL | 0.3171 mL | 0.3964 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
9-Dihydro-13-acetylbaccatin III (9-DHAB III) is an intermediate for taxol analog preparations. There are a series of closely related natural organic compounds isolated from the Pacific yew tree (Taxus brevifolia) and related species. Taxols have exhibit antitumor agents. 9-Dihydro-13-acetylbaccatin III is an antineoplastic agent and an anti-cancer intermediate.
- Taxumairol B
Catalog No.:BCN6940
CAS No.:142203-64-3
- KPT-276
Catalog No.:BCC4445
CAS No.:1421919-75-6
- GZD824
Catalog No.:BCC4389
CAS No.:1421783-64-3
- Hederacoside C
Catalog No.:BCN2329
CAS No.:14216-03-6
- Sweroside
Catalog No.:BCN6219
CAS No.:14215-86-2
- LY3039478
Catalog No.:BCC2105
CAS No.:1421438-81-4
- AZ5104
Catalog No.:BCC6389
CAS No.:1421373-98-9
- AZD-9291 mesylate
Catalog No.:BCC4121
CAS No.:1421373-66-1
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- Mutant EGFR inhibitor
Catalog No.:BCC4119
CAS No.:1421373-62-7
- Mutated EGFR-IN-1
Catalog No.:BCC5444
CAS No.:1421372-66-8
- CYM 50769
Catalog No.:BCC6337
CAS No.:1421365-63-0
- Catestatin
Catalog No.:BCC5935
CAS No.:142211-96-9
- Protionamide
Catalog No.:BCC4834
CAS No.:14222-60-7
- 2'-Rhamnoechinacoside
Catalog No.:BCN8219
CAS No.:1422390-59-7
- Rauvotetraphylline A
Catalog No.:BCN7052
CAS No.:1422506-49-7
- Rauvotetraphylline B
Catalog No.:BCN7056
CAS No.:1422506-50-0
- Rauvotetraphylline C
Catalog No.:BCN7055
CAS No.:1422506-51-1
- Rauvotetraphylline D
Catalog No.:BCN7054
CAS No.:1422506-52-2
- Rauvotetraphylline E
Catalog No.:BCN7051
CAS No.:1422506-53-3
- Kenpaullone
Catalog No.:BCC7047
CAS No.:142273-20-9
- Shizukaol B
Catalog No.:BCN6983
CAS No.:142279-40-1
- Shizukaol C
Catalog No.:BCN6225
CAS No.:142279-41-2
- Shizukaol D
Catalog No.:BCN6226
CAS No.:142279-42-3
Taxus canadensis abundant taxane: conversion to paclitaxel and rearrangements.[Pubmed:10896107]
Bioorg Med Chem. 2000 Jun;8(6):1269-80.
An efficient conversion of Taxus canadensis abundant taxane, 9-Dihydro-13-acetylbaccatin III to baccatin III is described. Since the synthesis of paclitaxel from baccatin III has been reported, this work can be used for additional supply of this powerful anticancer drug. In addition, new taxanes derived from skeletal rearrangements originating from oxidation reduction reactions of the Canadian yew major taxane, are reported.
Semi-synthesis of an O-glycosylated docetaxel analogue.[Pubmed:12628678]
Bioorg Med Chem. 2003 Apr 3;11(7):1551-6.
A 7beta-O-glycosylated docetaxel analogue was semi-synthesized from 9-Dihydro-13-acetylbaccatin III, the most abundant taxane isolated from the needles of Taxus canadensis. It was shown to be more bioactive than paclitaxel according to the tubulin assay. It had a reduced potency in the MCF7 cell line cytotoxicity assay compared to paclitaxel, but it demonstrated better activity against the drug resistant cell line MCF7-ADR. In addition, the presence of one sugar moiety on C-7 doubled the water solubility versus that of paclitaxel.


