LY3039478Notch inhibitor, novel and potent CAS# 1421438-81-4 |
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- FLI-06
Catalog No.:BCC5110
CAS No.:313967-18-9
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- MRK 560
Catalog No.:BCC2345
CAS No.:677772-84-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1421438-81-4 | SDF | Download SDF |
| PubChem ID | 71236992 | Appearance | Powder |
| Formula | C22H23F3N4O4 | M.Wt | 464.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Crenigacestat | ||
| Solubility | DMSO : ≥ 34 mg/mL (73.21 mM) *"≥" means soluble, but saturation unknown. | ||
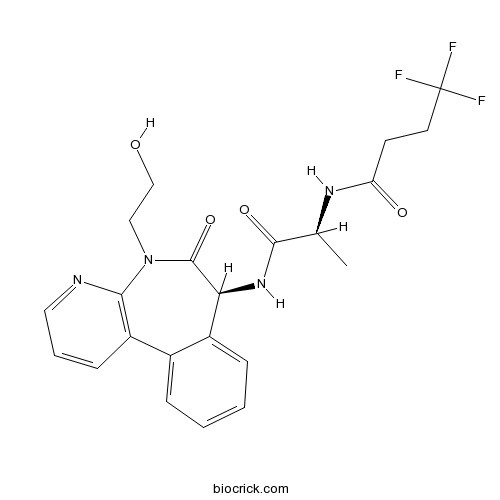
| Chemical Name | 4,4,4-trifluoro-N-[(2S)-1-[[(7S)-5-(2-hydroxyethyl)-6-oxo-7H-pyrido[2,3-d][3]benzazepin-7-yl]amino]-1-oxopropan-2-yl]butanamide | ||
| SMILES | CC(C(=O)NC1C2=CC=CC=C2C3=C(N=CC=C3)N(C1=O)CCO)NC(=O)CCC(F)(F)F | ||
| Standard InChIKey | YCBAQKQAINQRFW-UGSOOPFHSA-N | ||
| Standard InChI | InChI=1S/C22H23F3N4O4/c1-13(27-17(31)8-9-22(23,24)25)20(32)28-18-15-6-3-2-5-14(15)16-7-4-10-26-19(16)29(11-12-30)21(18)33/h2-7,10,13,18,30H,8-9,11-12H2,1H3,(H,27,31)(H,28,32)/t13-,18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY3039478 is a novel and potent Notch inhibitor. In a xenograft tumor model, LY3039478 inhibit N1ICD cleavage and expression of Notch-regulated genes in the tumor microenvironment. LY3039478 is being investigated in Phase I. |

LY3039478 Dilution Calculator

LY3039478 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1531 mL | 10.7657 mL | 21.5313 mL | 43.0626 mL | 53.8283 mL |
| 5 mM | 0.4306 mL | 2.1531 mL | 4.3063 mL | 8.6125 mL | 10.7657 mL |
| 10 mM | 0.2153 mL | 1.0766 mL | 2.1531 mL | 4.3063 mL | 5.3828 mL |
| 50 mM | 0.0431 mL | 0.2153 mL | 0.4306 mL | 0.8613 mL | 1.0766 mL |
| 100 mM | 0.0215 mL | 0.1077 mL | 0.2153 mL | 0.4306 mL | 0.5383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY3039478 is a novel and potent Notch inhibitor with IC50 of 0.41 nM.
The novel and unique Notch Inhibitor, LY3039478, has progressed into clinical development by Lilly co. ltd. LY3039478 displays the best overall profile and is unique within the SAR investigated. LY3039478 Displays Interesting Atropisomer Phenomena. The average LY3039478 major/minor rotational isomer ratio ranged between 14 and 28 in plasma. The crystalline monohydrate form of LY3039478 consists of a single rotational isomer and is chemically and physically stable for at least 14 days under accelerated stability test conditions.
Reference:
Warren J. Porter. Discovery of a Novel Notch Inhibitor. The 8th SCI-RSC Symposium on Proteinase Inhibitor Design April 15-16, 2013, Basel, Switzerland.
- AZ5104
Catalog No.:BCC6389
CAS No.:1421373-98-9
- AZD-9291 mesylate
Catalog No.:BCC4121
CAS No.:1421373-66-1
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- Mutant EGFR inhibitor
Catalog No.:BCC4119
CAS No.:1421373-62-7
- Mutated EGFR-IN-1
Catalog No.:BCC5444
CAS No.:1421372-66-8
- CYM 50769
Catalog No.:BCC6337
CAS No.:1421365-63-0
- (+)-Ketoconazole
Catalog No.:BCC4249
CAS No.:142128-59-4
- WS6
Catalog No.:BCC5566
CAS No.:1421227-53-3
- WS 3
Catalog No.:BCC7519
CAS No.:1421227-52-2
- Isosalvianolic acid C
Catalog No.:BCN3476
CAS No.:142115-17-1
- BIM 189
Catalog No.:BCC5934
CAS No.:142062-55-3
- Caproic acid
Catalog No.:BCC9218
CAS No.:142-62-1
- Sweroside
Catalog No.:BCN6219
CAS No.:14215-86-2
- Hederacoside C
Catalog No.:BCN2329
CAS No.:14216-03-6
- GZD824
Catalog No.:BCC4389
CAS No.:1421783-64-3
- KPT-276
Catalog No.:BCC4445
CAS No.:1421919-75-6
- Taxumairol B
Catalog No.:BCN6940
CAS No.:142203-64-3
- 9-Dihydro-13-acetylbaccatin III
Catalog No.:BCC1315
CAS No.:142203-65-4
- Catestatin
Catalog No.:BCC5935
CAS No.:142211-96-9
- Protionamide
Catalog No.:BCC4834
CAS No.:14222-60-7
- 2'-Rhamnoechinacoside
Catalog No.:BCN8219
CAS No.:1422390-59-7
- Rauvotetraphylline A
Catalog No.:BCN7052
CAS No.:1422506-49-7
- Rauvotetraphylline B
Catalog No.:BCN7056
CAS No.:1422506-50-0
- Rauvotetraphylline C
Catalog No.:BCN7055
CAS No.:1422506-51-1
Notch Inhibitor Shows Modest Efficacy.[Pubmed:27974417]
Cancer Discov. 2017 Feb;7(2):OF3.
Data from a phase I study indicate that LY3039478 is modestly effective against a range of advanced or metastatic cancers. The investigational Notch-signaling inhibitor induced partial responses and stable disease in patients with breast cancer and rare malignancies such as adenoid cystic carcinoma and leiomyosarcoma.
Notch Pathway Is Activated via Genetic and Epigenetic Alterations and Is a Therapeutic Target in Clear Cell Renal Cancer.[Pubmed:27909050]
J Biol Chem. 2017 Jan 20;292(3):837-846.
Clear cell renal cell carcinoma (CCRCC) is an incurable malignancy in advanced stages and needs newer therapeutic targets. Transcriptomic analysis of CCRCCs and matched microdissected renal tubular controls revealed overexpression of NOTCH ligands and receptors in tumor tissues. Examination of the TCGA RNA-seq data set also revealed widespread activation of NOTCH pathway in a large cohort of CCRCC samples. Samples with NOTCH pathway activation were also clinically distinct and were associated with better overall survival. Parallel DNA methylation and copy number analysis demonstrated that both genetic and epigenetic alterations led to NOTCH pathway activation in CCRCC. NOTCH ligand JAGGED1 was overexpressed and associated with loss of CpG methylation of H3K4me1-associated enhancer regions. JAGGED2 was also overexpressed and associated with gene amplification in distinct CCRCC samples. Transgenic expression of intracellular NOTCH1 in mice with tubule-specific deletion of VHL led to dysplastic hyperproliferation of tubular epithelial cells, confirming the procarcinogenic role of NOTCH in vivo Alteration of cell cycle pathways was seen in murine renal tubular cells with NOTCH overexpression, and molecular similarity to human tumors was observed, demonstrating that human CCRCC recapitulates features and gene expression changes observed in mice with transgenic overexpression of the Notch intracellular domain. Treatment with the gamma-secretase inhibitor LY3039478 led to inhibition of CCRCC cells in vitro and in vivo In summary, these data reveal the mechanistic basis of NOTCH pathway activation in CCRCC and demonstrate this pathway to a potential therapeutic target.


