NSC 625987Cyclin-dependent kinase 4 (cdk4) inhibitor CAS# 141992-47-4 |
- Batimastat (BB-94)
Catalog No.:BCC1223
CAS No.:130370-60-4
- GM 6001
Catalog No.:BCC2119
CAS No.:142880-36-2
- PD 166793
Catalog No.:BCC2376
CAS No.:199850-67-4
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
- WAY 170523
Catalog No.:BCC2380
CAS No.:307002-73-9
- ARP 100
Catalog No.:BCC2370
CAS No.:704888-90-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141992-47-4 | SDF | Download SDF |
| PubChem ID | 3004085 | Appearance | Powder |
| Formula | C15H13NO2S | M.Wt | 271.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in ethanol and to 100 mM in DMSO | ||
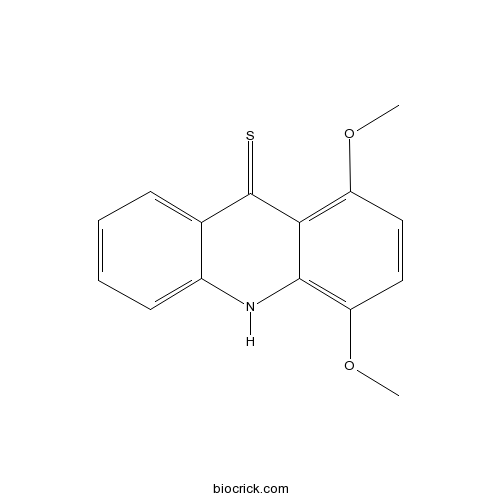
| Chemical Name | 1,4-dimethoxy-10H-acridine-9-thione | ||
| SMILES | COC1=C2C(=C(C=C1)OC)NC3=CC=CC=C3C2=S | ||
| Standard InChIKey | KFAKESMKRPNZTM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13NO2S/c1-17-11-7-8-12(18-2)14-13(11)15(19)9-5-3-4-6-10(9)16-14/h3-8H,1-2H3,(H,16,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclin-dependent kinase (cdk) 4 inhibitor (IC50 = 0.2 μM at cdk4/cyclin D1). Displays > 500-fold selectivity over cdk2 (IC50 > 100 μM for cdc2/cyclin A, cdk2/cyclin A and cdk2/cyclin E). |

NSC 625987 Dilution Calculator

NSC 625987 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6855 mL | 18.4277 mL | 36.8555 mL | 73.711 mL | 92.1387 mL |
| 5 mM | 0.7371 mL | 3.6855 mL | 7.3711 mL | 14.7422 mL | 18.4277 mL |
| 10 mM | 0.3686 mL | 1.8428 mL | 3.6855 mL | 7.3711 mL | 9.2139 mL |
| 50 mM | 0.0737 mL | 0.3686 mL | 0.7371 mL | 1.4742 mL | 1.8428 mL |
| 100 mM | 0.0369 mL | 0.1843 mL | 0.3686 mL | 0.7371 mL | 0.9214 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl 4-Hydroxyphenylacetate
Catalog No.:BCN1571
CAS No.:14199-15-6
- Emetine Hydrochloride
Catalog No.:BCN2478
CAS No.:14198-59-5
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
- Ginsenoside Rg3
Catalog No.:BCN1068
CAS No.:14197-60-5
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- 4-Hydroxyphenylacetonitrile
Catalog No.:BCN6905
CAS No.:14191-95-8
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- EI1
Catalog No.:BCC4044
CAS No.:1418308-27-6
- NVP-TNKS656
Catalog No.:BCC6541
CAS No.:1419949-20-4
- Caproic acid
Catalog No.:BCC9218
CAS No.:142-62-1
- BIM 189
Catalog No.:BCC5934
CAS No.:142062-55-3
- Isosalvianolic acid C
Catalog No.:BCN3476
CAS No.:142115-17-1
- WS 3
Catalog No.:BCC7519
CAS No.:1421227-52-2
- WS6
Catalog No.:BCC5566
CAS No.:1421227-53-3
- (+)-Ketoconazole
Catalog No.:BCC4249
CAS No.:142128-59-4
- CYM 50769
Catalog No.:BCC6337
CAS No.:1421365-63-0
- Mutated EGFR-IN-1
Catalog No.:BCC5444
CAS No.:1421372-66-8
- Mutant EGFR inhibitor
Catalog No.:BCC4119
CAS No.:1421373-62-7
- AZD-9291
Catalog No.:BCC4120
CAS No.:1421373-65-0
- AZD-9291 mesylate
Catalog No.:BCC4121
CAS No.:1421373-66-1
VEGF alleviates ALS-CSF induced cytoplasmic accumulations of TDP-43 and FUS/TLS in NSC-34 cells.[Pubmed:28163215]
J Chem Neuroanat. 2017 Apr;81:48-52.
Cytoplasmic mislocalisation and aggregation of TDP-43 and FUS/TLS proteins in spinal motor neurons contribute to the pathogenesis of the highly fatal disorder amyotrophic lateral sclerosis (ALS). We investigated the neuroprotective effect of VEGF on expression of these proteins in the motor neuronal cell line NSC-34 modelled to reminisce sporadic form of ALS. We studied the expression of TDP-43 and FUS/TLS proteins after exposure to ALS-CSF and following VEGF supplementation by quantitative confocal microscopy and electron microscopy. ALS-CSF caused cytoplasmic overexpression of both the proteins and stress-granule formation in the cells. These alterations were alleviated by VEGF supplementation. The related ultrastructural changes like nuclear membrane dysmorphism and p-bodies associated changes were also reversed. However the protein expression did not completely translocate to the nucleus, as some cells continued to show to cytoplasmic mislocalisation. Thus, the present findings indicate that VEGF alleviates TDP43 and FUS pathology by complimenting its role in controlling apoptosis and reversing choline acetyl transferase expression. Hence, VEGF appears to target multiple pathogenic processes in the neurodegenerative cascade of ALS.
Important modifications by sugammadex, a modified gamma-cyclodextrin, of ion currents in differentiated NSC-34 neuronal cells.[Pubmed:28049438]
BMC Neurosci. 2017 Jan 3;18(1):6.
BACKGROUND: Sugammadex (SGX) is a modified gamma-cyclodextrin used for reversal of steroidal neuromuscular blocking agents during general anesthesia. Despite its application in clinical use, whether SGX treatment exerts any effects on membrane ion currents in neurons remains largely unclear. In this study, effects of SGX treatment on ion currents, particularly on delayed-rectifier K(+) current [I K(DR)], were extensively investigated in differentiated NSC-34 neuronal cells. RESULTS: After cells were exposed to SGX (30 muM), there was a reduction in the amplitude of I K(DR) followed by an apparent slowing in current activation in response to membrane depolarization. The challenge of cells with SGX produced a depolarized shift by 15 mV in the activation curve of I K(DR) accompanied by increased gating charge of this current. However, the inactivation curve of I K(DR) remained unchanged following SGX treatment, as compared with that in untreated cells. According to a minimal reaction scheme, the lengthening of activation time constant of I K(DR) caused by cell treatment with different SGX concentrations was quantitatively estimated with a dissociation constant of 17.5 muM, a value that is clinically achievable. Accumulative slowing in I K(DR) activation elicited by repetitive stimuli was enhanced in SGX-treated cells. SGX treatment did not alter the amplitude of voltage-gated Na(+) currents. In SGX-treated cells, dexamethasone (30 muM), a synthetic glucocorticoid, produced little or no effect on L-type Ca(2+) currents, although it effectively suppressed the amplitude of this current in untreated cells. CONCLUSIONS: The treatment of SGX may influence the amplitude and gating of I K(DR) and its actions could potentially contribute to functional activities of motor neurons if similar results were found in vivo.
Adult NSC diversity and plasticity: the role of the niche.[Pubmed:27978480]
Curr Opin Neurobiol. 2017 Feb;42:68-74.
Adult somatic stem cells are generally defined as cells with the ability to differentiate into multiple different lineages and to self-renew during long periods of time. These features were long presumed to be represented in one single tissue-specific stem cell. Recent development of single-cell technologies reveals the existence of diversity in fate and activation state of somatic stem cells within the blood, skin and intestinal compartments [1] but also in the adult brain. Here we review how recent advances have expanded our view of neural stem cells (NSCs) as a diverse pool of cells and how the specialized microenvironment in which they reside acts to maintain this diversity. In addition, we discuss the plasticity of the system in the injured brain.
c-Jun Amino-Terminal Kinase is Involved in Valproic Acid-Mediated Neuronal Differentiation of Mouse Embryonic NSCs and Neurite Outgrowth of NSC-Derived Neurons.[Pubmed:28321599]
Neurochem Res. 2017 Apr;42(4):1254-1266.
Valproic acid (VPA), an anticonvulsant and mood-stabilizing drug, can induce neuronal differentiation, promote neurite extension and exert a neuroprotective effect in central nervous system (CNS) injuries; however, comparatively little is known regarding its action on mouse embryonic neural stem cells (NSCs) and the underlying molecular mechanism. Recent studies suggested that c-Jun N-terminal kinase (JNK) is required for neurite outgrowth and neuronal differentiation during neuronal development. In the present study, we cultured mouse embryonic NSCs and treated the cells with 1 mM VPA for up to 7 days. The results indicate that VPA promotes the neuronal differentiation of mouse embryonic NSCs and neurite outgrowth of NSC-derived neurons; moreover, VPA induces the phosphorylation of c-Jun by JNK. In contrast, the specific JNK inhibitor SP600125 decreased the VPA-stimulated increase in neuronal differentiation of mouse embryonic NSCs and neurite outgrowth of NSC-derived neurons. Taken together, these results suggest that VPA promotes neuronal differentiation of mouse embryonic NSCs and neurite outgrowth of NSC-derived neurons. Moreover, JNK activation is involved in the effects of VPA stimulation.
Structural determinants of CDK4 inhibition and design of selective ATP competitive inhibitors.[Pubmed:15123247]
Chem Biol. 2004 Apr;11(4):525-34.
A number of selective inhibitors of the CDK4/cyclin D1 complex have been reported recently. Due to the absence of an experimental CDK4 structure, the ligand and protein determinants contributing to CDK4 selectivity are poorly understood at present. Here, we report the use of computational methods to elucidate the characteristics of selectivity and to derive the structural basis for specific, high-affinity binding of inhibitors to the CDK4 active site. From these data, the hypothesis emerged that appropriate incorporation of an ionizable function into a CDK2 inhibitor results in more favorable binding to CDK4. This knowledge was applied to the design of compounds in the otherwise CDK2-selective 2-anilino-4-(thiazol-5-yl)pyrimidine pharmacophore that are potent and highly selective ATP antagonists of CDK4/cyclin D1. The findings of this study also have significant implications in the design of CDK4 mimic structures based on CDK2.
The p16 status of tumor cell lines identifies small molecule inhibitors specific for cyclin-dependent kinase 4.[Pubmed:10632371]
Clin Cancer Res. 1999 Dec;5(12):4279-86.
Loss of p16 functional activity leading to disruption of the p16/cyclin-dependent kinase (CDK) 4:cyclin D/retinoblastoma pathway is the most common event in human tumorigenesis, suggesting that compounds with CDK4 kinase inhibitory activity may be useful to regulate cancer cell growth. To identify such inhibitors, the 60 cancer cell lines of the National Cancer Institute drug screen panel were examined for p16 alterations (biallelic deletion, intragenic mutations, or absent p16 protein), and the growth-inhibitory activity of more than 50,000 compounds against these 60 cell lines was compared with their p16 status. One compound, 3-amino thioacridone (3-ATA; NSC 680434), whose growth-inhibitory activity correlated with the p16 status of the cell lines had an IC50 of 3.1 microM in a CDK4 kinase assay. In addition, four compounds structurally related to 3-ATA inhibited CDK4 kinase with IC50s ranging from 0.2-2.0 microM. All five of these compounds were less potent inhibitors of cell division cycle 2 and CDK2 kinases, with IC50s 30- to 500-fold higher than that for CDK4. ATP competition experiments demonstrated a noncompetitive mode of inhibition for 3-ATA (K(i) = 5.5 microM) and a linear mixed mode for benzothiadiazine (NSC 645787; K(i) = 0.73 microM). We have successfully demonstrated a novel approach to identify specific CDK4 kinase inhibitors that may selectively induce growth inhibition of p16-altered tumors.


