4-HydroxyphenylacetonitrileCAS# 14191-95-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14191-95-8 | SDF | Download SDF |
| PubChem ID | 26548 | Appearance | Powder |
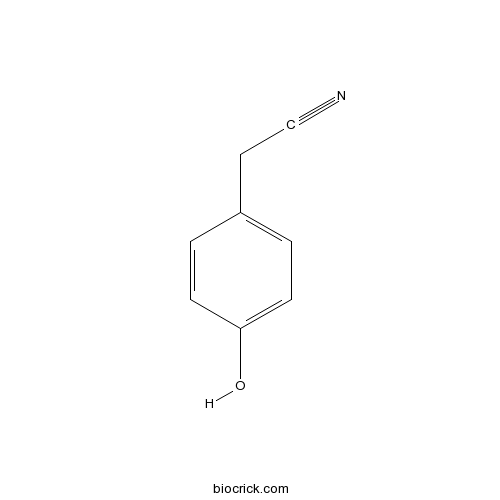
| Formula | C8H7NO | M.Wt | 133.15 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(4-hydroxyphenyl)acetonitrile | ||
| SMILES | C1=CC(=CC=C1CC#N)O | ||
| Standard InChIKey | AYKYOOPFBCOXSL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H7NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 4-Hydroxyphenylacetonitrile shows strong antioxidative activity. |

4-Hydroxyphenylacetonitrile Dilution Calculator

4-Hydroxyphenylacetonitrile Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.5103 mL | 37.5516 mL | 75.1033 mL | 150.2065 mL | 187.7582 mL |
| 5 mM | 1.5021 mL | 7.5103 mL | 15.0207 mL | 30.0413 mL | 37.5516 mL |
| 10 mM | 0.751 mL | 3.7552 mL | 7.5103 mL | 15.0207 mL | 18.7758 mL |
| 50 mM | 0.1502 mL | 0.751 mL | 1.5021 mL | 3.0041 mL | 3.7552 mL |
| 100 mM | 0.0751 mL | 0.3755 mL | 0.751 mL | 1.5021 mL | 1.8776 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-HoTyr-OH.HBr
Catalog No.:BCC3245
CAS No.:141899-12-9
- Fmoc-Cys(pMeOBzl)-OH
Catalog No.:BCC3477
CAS No.:141892-41-3
- Magnoloside D
Catalog No.:BCN8062
CAS No.:1418309-03-1
- EI1
Catalog No.:BCC4044
CAS No.:1418308-27-6
- Minaxin C
Catalog No.:BCN7656
CAS No.:1418150-06-7
- LMK 235
Catalog No.:BCC2421
CAS No.:1418033-25-6
- NMS-873
Catalog No.:BCC4977
CAS No.:1418013-75-8
- Endomorphin-2
Catalog No.:BCC5697
CAS No.:141801-26-5
- Stauntosaponin A
Catalog No.:BCN6966
CAS No.:1417887-91-2
- Albatrelin C
Catalog No.:BCN7591
CAS No.:1417805-17-4
- Albatrelin A
Catalog No.:BCN7626
CAS No.:1417805-15-2
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- Pimentol
Catalog No.:BCN2714
CAS No.:141913-95-3
- Ginsenoyne K
Catalog No.:BCN3953
CAS No.:141947-42-4
- Ginsenoside Rg3
Catalog No.:BCN1068
CAS No.:14197-60-5
- 14-Deoxy-11,12-didehydroandrographiside
Catalog No.:BCN1572
CAS No.:141973-41-3
- 16-Hydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN7500
CAS No.:141979-19-3
- Emetine Hydrochloride
Catalog No.:BCN2478
CAS No.:14198-59-5
- Methyl 4-Hydroxyphenylacetate
Catalog No.:BCN1571
CAS No.:14199-15-6
- NSC 625987
Catalog No.:BCC7269
CAS No.:141992-47-4
- NVP-TNKS656
Catalog No.:BCC6541
CAS No.:1419949-20-4
- Caproic acid
Catalog No.:BCC9218
CAS No.:142-62-1
- BIM 189
Catalog No.:BCC5934
CAS No.:142062-55-3
- Isosalvianolic acid C
Catalog No.:BCN3476
CAS No.:142115-17-1
Novel phenolic glycosides, adenophorasides A-E, from Adenophora roots.[Pubmed:20229365]
J Nat Med. 2010 Jul;64(3):245-51.
Five novel phenolic glycosides, adenophorasides A (1), B (2), C (3), D (4), and E (5), were isolated from commercial Adenophora roots, together with vanilloloside (6), 3,4-dimethoxybenzyl alcohol 7-O-beta-D: -glucopyranoside (7), and lobetyolin (8). The structures of the new compounds (1-5) were characterized as 4-hydroxy-3-methoxyphenylacetonitrile 4-O-beta-D: -glucopyranoside (1), 4-hydroxy-3-methoxyphenylacetonitrile 4-O-beta-D: -glucopyranosyl-(1-->6)-beta-D: -glucopyranoside (2), 4-hydroxy-3-methoxyphenylacetonitrile 4-O-alpha-L: -rhamnopyranosyl-(1-->6)-beta-D: -glucopyranoside (3), 4-Hydroxyphenylacetonitrile 4-O-beta-D: -glucopyranosyl-(1-->6)-beta-D: -glucopyranoside (4), and 4-hydroxy-3-methoxybenzyl alcohol 4-O-beta-D: -glucopyranosyl-(1-->6)-beta-D: -glucopyranoside (5), respectively, by means of spectroscopic and chemical analyses.
Two new bromotyrosine-derived metabolites from the sponge Psammaplysilla purpurea.[Pubmed:12913243]
Chem Pharm Bull (Tokyo). 2003 Aug;51(8):990-3.
Two new bromotyrosine-derived metabolites (1, 2) have been isolated along with the known compounds 3,5-dibromo-4-methoxyphenylacetonitrile, 3-bromo-4-methoxyphenylacetonitrile, 3-bromo-4-Hydroxyphenylacetonitrile, 1-hydroxyuracil, 1-methoxyhemibastadin 2, purpuramine H and a steroid 5alpha,8alpha-epidioxycholest-6-en-3beta-ol from the sponge Psammaplysilla purpurea. Compounds 1 and 2 were characterized by interpretation of their spectral data. The antibacterial activity of these compounds is summarized.
Sinapis phylogeny and evolution of glucosinolates and specific nitrile degrading enzymes.[Pubmed:18995873]
Phytochemistry. 2008 Dec;69(17):2937-49.
Levels of sinalbin (4-hydroxybenzylglucosinolate) and 28 other glucosinolates were determined in leaves and roots of 20 species that were either phylogenetically close to Sinapis alba, Sinapis arvensis, or Sinapis pubescens (tribe Brassiceae, Brassicaceae), or were expected to contain arylalkyl nitrilase activity. Comparison with a molecular phylogenetic tree based on ITS DNA sequences identified two separate occurrences of sinalbin. The first in a group of species related to S. alba (including members of the genera Coincya and Kremeriella); and the second in S. arvensis, nested among sinalbin deficient species. Significant 4-Hydroxyphenylacetonitrile degrading enzyme activity was found in both S. alba and S. arvensis, but in S. alba the major product was the corresponding carboxylic acid, while in S. arvensis the major product was the amide. Both investigated enzyme activities, nitrilase and nitrile hydratase, were specific, accepting only certain arylacetonitriles such as 4-hydroxy and 4-methoxyphenylacetonitrile. Only the S. alba enzyme required an oxygen in para position of the substrate, as found in sinalbin. Indole-3-acetonitrile, arylcyanides, and arylpropionitriles were poor substrates. The nitrilase activity of S. alba was quantitatively comparable to that reported in the monocot Sorghum bicolor (believed to be involved in cyanogenic glycoside metabolism). Glucosinolates derived from methionine were found in all Sinapis clades. Glucosinolate patterns suggested a complex evolution of glucosinolates in the investigated species, with several apparent examples of abrupt changes in glucosinolate profiles including chain length variation and appearance of glucosinolates derived from branched-chain amino acids. NMR data for desulfated homosinalbin, 9-methylsulphonylnonylglucosinolate, 3-methylpentylglucosinolate and related glucosinolates are reported, and a facultative connection between sinalbin and specific nitrilases is suggested.
Host plant-dependent metabolism of 4-hydroxybenzylglucosinolate in Pieris rapae: substrate specificity and effects of genetic modification and plant nitrile hydratase.[Pubmed:17916498]
Insect Biochem Mol Biol. 2007 Nov;37(11):1119-30.
After ingestion of transgenic Arabidopsis thaliana CYP79A1 containing sinalbin (4-hydroxybenzylglucosinolate) due to genetic modification, only one major sinalbin-derived sulphate ester (the sulphate ester of 4-Hydroxyphenylacetonitrile) was excreted by Pieris rapae caterpillars (corresponding to 69mol% of ingested sinalbin). An additional sulphate ester (the sulphate ester of 4-hydroxyphenylacetamide) was excreted when the caterpillars were reared on two plant species (Sinapis alba and Sinapis arvensis) that contained sinalbin naturally. Artificial addition of sinalbin to S. arvensis leaves resulted in increased levels of the sulphated amide, and an enzymatic activity (nitrile hydratase) explaining the formation of the sulphated amide from sinalbin was detected in both Sinapis species, but not in A. thaliana. In agreement with the suggested minor metabolic pathway, the caterpillars were able to sulphate 4-hydroxyphenylacetamide offered as part of an artificial diet. In fact, phenol and seven para-substituted phenol derivatives with substituents of moderate size were sulphated and excreted, but all tested phenols devoid of a nitrile functional group were less efficiently sulphated than the primary sinalbin detoxification product, 4-Hydroxyphenylacetonitrile. This suggests that the specificity of the sulphation step involved in sinalbin metabolism may be adapted to nitriles formed as metabolites of phenolic glucosinolates. On the contrary, there was no specificity for products (4-hydroxybenzylascorbigen and 4-hydroxybenzylalcohol) derived from the semistable isothiocyanate produced from sinalbin in the absence of nitrile specifier protein.
Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism.[Pubmed:18003897]
Proc Natl Acad Sci U S A. 2007 Nov 20;104(47):18848-53.
Members of the nitrilase 4 (NIT4) family of higher plants catalyze the conversion of beta-cyanoalanine to aspartic acid and asparagine, a key step in cyanide detoxification. Grasses (Poaceae) possess two different NIT4 homologs (NIT4A and NIT4B), but none of the recombinant Poaceae enzymes analyzed showed activity with beta-cyanoalanine, whereas protein extracts of the same plants clearly posses this activity. Sorghum bicolor contains three NIT4 isoforms SbNIT4A, SbNIT4B1, and SbNIT4B2. Individually, each isoform does not possess enzymatic activity whereas the heteromeric complexes SbNIT4A/B1 and SbNIT4A/B2 hydrolyze beta-cyanoalanine with high activity. In addition, the SbNIT4A/B2 complex accepts additional substrates, the best being 4-Hydroxyphenylacetonitrile. Corresponding NIT4A and NIT4B isoforms from other Poaceae species can functionally complement the sorghum isoforms in these complexes. Site-specific mutagenesis of the active site cysteine residue demonstrates that hydrolysis of beta-cyanoalanine is catalyzed by the NIT4A isoform in both complexes whereas hydrolysis of 4-Hydroxyphenylacetonitrile occurs at the NIT4B2 isoform. 4-Hydroxyphenylacetonitrile was shown to be an in vitro breakdown product of the cyanogenic glycoside dhurrin, a main constituent in S. bicolor. The results indicate that the SbNIT4A/B2 heterocomplex plays a key role in an endogenous turnover of dhurrin proceeding via 4-Hydroxyphenylacetonitrile and thereby avoiding release of toxic hydrogen cyanide. The operation of this pathway would enable plants to use cyanogenic glycosides as transportable and remobilizable nitrogenous storage compounds. Through combinatorial biochemistry and neofunctionalizations, the small family of nitrilases has gained diverse biological functions in nitrile metabolism.
Antioxidants from rape (Brassica campestris vir. Japonica Hara) oil cake.[Pubmed:15143833]
Nat Prod Res. 2004 Jun;18(3):231-9.
A simple but novel compound, S-1-methoxy-1-(3,5-dimethoxy-4-hydroxyphenyl)ethane, was isolated as a moderately antioxidative compound from rape (Brassica campestris L. subsp. napus) oil cake together with 5 known compounds. Three of these compounds, indolacetonitrile, 4-hydroxyindolacetonitrile, and 4-Hydroxyphenylacetonitrile, showed strong antioxidative activity evaluated by the ferric thiocyanate method.
Aspects on the Biosynthesis of the Cyanogenic Glucoside Triglochinin in Triglochin maritima1.[Pubmed:17340339]
Planta Med. 1984 Oct;50(5):394-7.
The incorporation of L-[U- (14)C]phenylalanine, L-[U- (14)C]tyrosine and [U- (14)C]4-Hydroxyphenylacetonitrile into triglochinin and taxiphyllin, the latter a possible precursor of the former, was studied in seedlings of TRIGLOCHIN MARITIMA L. The nitrile was by far the best substrate; incorporation of the amino acids was poor. Environmental factors such as light and humidity act differently on production of both compounds which also show a wide variation in individual seedlings. Quantitative evaluation of the specific activities indicate that taxiphyllin cannot be regarded as a precursor of triglochinin and that the two compounds are probably synthesized by two, at least mainly independent, pathways.
A simple, selective, and sensitive gas chromatography-mass spectrometry method for the analysis of five process-related impurities in atenolol bulk drug and capsule formulations.[Pubmed:28581679]
J Sep Sci. 2017 Aug;40(15):3086-3093.
An extremely sensitive and simple gas chromatography with mass spectrometry method was developed and completely validated for the analysis of five process-related impurities, viz., 4-hydroxy-l-phenylglycine, 4-Hydroxyphenylacetonitrile, 4-hydroxyphenylacetic acid, methyl-4-hydroxyphenylacetate, and 2-[4-{(2RS)-2-hydroxy-3-[(1-methylethyl)amino]propoxy}phenyl]acetonitrile, in atenolol. The separation of impurities was accomplished on a BPX-5 column with dimensions of 50 m x 0.25 mm i.d. and 0.25 mum film thickness. The method validation was performed following International Conference on Harmonisation guidelines in which the method was capable to quantitate 4-hydroxy-l-phenylglycine, 4-Hydroxyphenylacetonitrile, and 4-hydroxyphenylacetic acid at 0.3 ppm, and methyl-4-hydroxyphenylacetate and 2-[4-{(2RS)-2-hydroxy-3-[(1-methylethyl)amino]propoxy}phenyl]acetonitrile at 0.35 ppm with respect to 10 mg/mL of atenolol. The method was linear over the concentration range of 0.3-10 ppm for 4-hydroxy-l-phenylglycine, 4-Hydroxyphenylacetonitrile, and 4-hydroxyphenylacetic acid, and 0.35-10 ppm for methyl-4-hydroxyphenylacetate and 2-[4-{(2RS)-2-hydroxy-3-[(1-methylethyl)amino]propoxy}phenyl]acetonitrile. The correlation coefficient in each case was found >/=0.998. The repeatability and recovery values were acceptable, and found between 89.38% and 105.60% for all five impurities under optimized operating conditions. The method developed here is simple, selective, and sensitive with apparently better resolution than the reported methods. Hence, the method is a straightforward and good quality control tool for the quantitation of selected impurities at trace concentrations in atenolol.


