SP2509Demethylase 1 (LSD1) antagonist, novel Lysine-specific CAS# 1423715-09-6 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- AEG 3482
Catalog No.:BCC8088
CAS No.:63735-71-7
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1423715-09-6 | SDF | Download SDF |
| PubChem ID | 71494974 | Appearance | Powder |
| Formula | C19H20ClN3O5S | M.Wt | 437.90 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 33 mg/mL (75.36 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
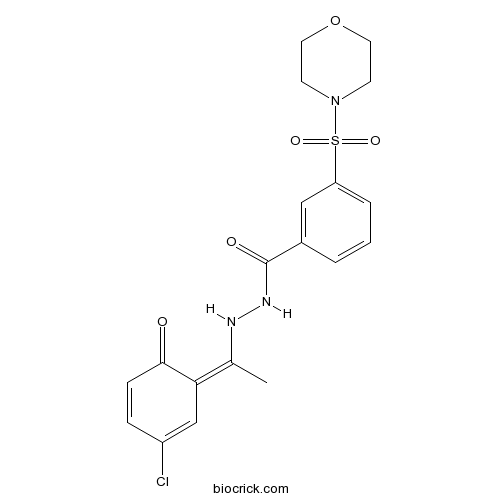
| Chemical Name | N'-[(1Z)-1-(3-chloro-6-oxocyclohexa-2,4-dien-1-ylidene)ethyl]-3-morpholin-4-ylsulfonylbenzohydrazide | ||
| SMILES | CC(=C1C=C(C=CC1=O)Cl)NNC(=O)C2=CC(=CC=C2)S(=O)(=O)N3CCOCC3 | ||
| Standard InChIKey | ZVVCYRYDHNYAPV-LGMDPLHJSA-N | ||
| Standard InChI | InChI=1S/C19H20ClN3O5S/c1-13(17-12-15(20)5-6-18(17)24)21-22-19(25)14-3-2-4-16(11-14)29(26,27)23-7-9-28-10-8-23/h2-6,11-12,21H,7-10H2,1H3,(H,22,25)/b17-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SP2509 is a potent and selective antagonist of lysine specific demethylase 1 (LSD1) with IC50 of 13 nM.In Vitro:SP2509 (250, 500, 1000 nM) inhibits LSD1 activity, depletes colony growth and induces apoptosis and cell death of cultured human acute myeloid leukemia cells, and increases H3K4Me3 on the promoters of p57 Kip, KLF4, and p21 and induces mRNA expression of p57Kip, KLF4 and p21 in AML cells. SP2509 (250, 1000 nM) induces features of morphologic differentiation of cultured and primary AML cells. Besides, SP2509 in combination with PS exerts synergistic lethal activity against cultured and primary AML cells[1]. SP2509 does not destabilize the CoREST-LSD1 interaction, and has no major destabilizing effect on the CRC. SP2509 (1 or 10 µM) induces cell death, but there are no morphological changes at a low concertation of 0.1 µM. SP2509 likewise interferes with the viability of medulloblastoma cells[2].In Vivo:Treatment with SP2509 (25 mg/kg) and/or PS (5 mg/kg) significantly enhances PS-mediated loss of viability of CD34+ primary AML cells and improves the survival of mice bearing AML xenografts and primagrafts[2]. References: | |||||
| Kinase experiment [1]: | |
| SP2509 activity assay | Test compounds were diluted to 20 × the desired test concentration in 100% dimethyl sulfoxide and 2.5 μl of the diluted drug sample was added to a black 384-well plate. The LSD1 enzyme stock was diluted 17-fold with assay buffer and 40 μl of the diluted LSD1 enzyme was added to the appropriate wells. Substrate, consisting of horseradish peroxidase, dimethyl K4 peptide corresponding to the first 21 amino acids of the N-terminal tail of histone H3, and 10-acetyl-3,7-dihydroxyphenoxazine was then added to wells. Resorufin was analyzed on a plate reader with an excitation wavelength of 530 nm and an emission wavelength of 595 nm. |
| Cell experiment [1]: | |
| Cell lines | OCI-AML3 and MOLM13 |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 16 h |
| Applications | SP2509 inhibits LSD1 activity, reduces colony growth and induces apoptosis of cultured AML cells. SP2509 treatment also inhibits the association of LSD1 with CoREST, increases promoter-specific H3K4Me3 and induces p53, p21 and C/EBPα in AML cells. Furthermore, treatment with SP2509 induces differentiation of cultured and primary AML cells |
| Animal experiment [1]: | |
| Animal models | NOD/SCID mice |
| Dosage form | 25 mg/kg SP2509 administered twice per week (Tuesday and Thursday) intraperitoneally for 3 weeks |
| Application | Treatment with either SP2509 improves the median survival of the mice infused with OCI-AML3 cells (37 and 36.5 days, respectively, versus 19.5 days for vehicle control). Notably, combined treatment with panobinostat and SP2509 further improves the median survival of the mice (44.5 days), as compared with treatment with SP2509 or panobinostat alone (P<0.01). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Fiskus W, Sharma S, Shah B et al. Highly effective combination of LSD1 (KDM1A) antagonist andpan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014 Nov;28(11):2155-64 | |

SP2509 Dilution Calculator

SP2509 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2836 mL | 11.4181 mL | 22.8363 mL | 45.6725 mL | 57.0907 mL |
| 5 mM | 0.4567 mL | 2.2836 mL | 4.5673 mL | 9.1345 mL | 11.4181 mL |
| 10 mM | 0.2284 mL | 1.1418 mL | 2.2836 mL | 4.5673 mL | 5.7091 mL |
| 50 mM | 0.0457 mL | 0.2284 mL | 0.4567 mL | 0.9135 mL | 1.1418 mL |
| 100 mM | 0.0228 mL | 0.1142 mL | 0.2284 mL | 0.4567 mL | 0.5709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SP2509 is a novel Lysine specific demethylase 1 (LSD1) antagonist (IC50 = 13nM) with no effect on MAO-A and MAO-B. [1]
LSD1 demethylates mono-/di-methylated lysine 4 on histone H3. High expression of LSD1 is associated with poor prognosis in cancer. [2]
In cultured human AML cells, SP2590 inhibited LSD1 activity, deplete colony growth and elicit apoptosis. SP2590 also inhibited the LSD1 binding to CoREST, increase promoter-specific H3K4Me3 and induces p53, p21 and C/EBPα in AML cells. Moreover, SP2590 promotes differentiated of cultured and primary AML cell. [1]
In mice carrying AML xenografts, SP2590 treatment alone significantly improved the survival of mice and co-treatment with an-HDAC inhibitor (HDI) panobinostat (PS) exhibited superior in vivo activity. [1]
References:
1. Fiskus W, Sharma S, Shah B, Portier BP, Devaraj SG, Liu K, Iyer SP, Bearss D, Bhalla KN. Highly effective combination of LSD1 (KDM1A) antagonist andpan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014 Nov;28(11):2155-64.
2. Zhao ZK, Yu HF, Wang DR, Dong P, Chen L, Wu WG, Ding WJ, Liu YB.
Overexpression of lysine specific demethylase 1 predicts worse prognosis in primary hepatocellular carcinoma patients. World J Gastroenterol. 2012 Dec 7;18(45):6651-6.
- H-D-Phe-pNA
Catalog No.:BCC3015
CAS No.:14235-18-8
- Adefovir Dipivoxil
Catalog No.:BCC5025
CAS No.:142340-99-6
- L-701,324
Catalog No.:BCC6842
CAS No.:142326-59-8
- Teuvincenone H
Catalog No.:BCN6227
CAS No.:142299-73-8
- Shizukaol D
Catalog No.:BCN6226
CAS No.:142279-42-3
- Shizukaol C
Catalog No.:BCN6225
CAS No.:142279-41-2
- Shizukaol B
Catalog No.:BCN6983
CAS No.:142279-40-1
- Kenpaullone
Catalog No.:BCC7047
CAS No.:142273-20-9
- Rauvotetraphylline E
Catalog No.:BCN7051
CAS No.:1422506-53-3
- Rauvotetraphylline D
Catalog No.:BCN7054
CAS No.:1422506-52-2
- Rauvotetraphylline C
Catalog No.:BCN7055
CAS No.:1422506-51-1
- Rauvotetraphylline B
Catalog No.:BCN7056
CAS No.:1422506-50-0
- FR 139317
Catalog No.:BCC5733
CAS No.:142375-60-8
- 7alpha-Hydroxy-4,11-cadinadiene-3,8-dione
Catalog No.:BCN7057
CAS No.:1423809-64-6
- Didemethylpseudoaspidin AA
Catalog No.:BCN3777
CAS No.:142382-28-3
- Mesterolone
Catalog No.:BCC9023
CAS No.:1424-00-6
- Crovatin
Catalog No.:BCN2517
CAS No.:142409-09-4
- NHS-SS-Biotin
Catalog No.:BCC3581
CAS No.:142439-92-7
- Myricetin 3-O-beta-D-xylopyranosyl(1-2)-beta-D-glucopyranoside
Catalog No.:BCN8140
CAS No.:142449-93-2
- Lobetyolinin
Catalog No.:BCN3322
CAS No.:142451-48-7
- Amthamine dihydrobromide
Catalog No.:BCC6744
CAS No.:142457-00-9
- 3-O-beta-D-apiofuranosyl(1-2)-beta-D-glucopyranosyl rhamnocitrin 4-O-beta-D-glucopyranoside
Catalog No.:BCN8141
CAS No.:142473-99-2
- Glyasperin A
Catalog No.:BCN6228
CAS No.:142474-52-0
- 19-Nortestosterone acetate
Catalog No.:BCC8445
CAS No.:1425-10-1
Suppression of LSD1 enhances the cytotoxic and apoptotic effects of regorafenib in hepatocellular carcinoma cells.[Pubmed:30929918]
Biochem Biophys Res Commun. 2019 Mar 28. pii: S0006-291X(19)30554-6.
Regorafenib has been approved to treat patients who have HCC progression after sorafenib failure, however, regorafenib also faces the risk of drug resistance and subsequent progression of HCC patients. As LSD1 inhibitors can alleviate acquired resistance to sorafenib, in this context, we are interested to investigate the role of LSD1 in regorafenib treatment. Firstly, over-expressed LSD1 was observed in HCC patients and predicted poor prognosis. However, regorafenib failed to suppress the expression of LSD1 in HCC cells. Thus, we hypothesized that LSD1 inhibition could enhance the anti-HCC activity of regorafenib. As expected, LSD1 knockdown could enhance anti-proliferation effect of regorafenib in HCC cells. LSD1 inhibitor SP2509 could enhance the cytotoxic and apoptotic effects of regorafenib in HCC cells. In addition, clinically used LSD1 inhibitor tranylcypromine also enhanced anti-HCC effect of regorafenib. Furthermore, LSD1 suppressed by SP2590 or tranylcypromine could alleviate the activated p-AKT (ser473) induced by regorafenib in HCC cells. Thus, inhibiting LSD1 might be an attractive target for regorafenib sensitization and clinical HCC therapy, our findings could help to elucidate more effective therapeutic options for HCC patients.
Hypoxia Promotes Resistance to EGFR Inhibition in NSCLC Cells via the Histone Demethylases, LSD1 and PLU-1.[Pubmed:29934325]
Mol Cancer Res. 2018 Oct;16(10):1458-1469.
The development of small-molecule tyrosine kinase inhibitors (TKI) specific for epidermal growth factor receptors (EGFR) with activating mutations has led to a new paradigm in the treatment of non-small cell lung cancer (NSCLC) patients. However, most patients eventually develop resistance. Hypoxia is a key microenvironmental stress in solid tumors that is associated with poor prognosis due, in part, to acquired resistance to conventional therapy. This study documents that long-term, moderate hypoxia promotes resistance to the EGFR TKI, gefitinib, in the NSCLC cell line HCC827, which harbors an activating EGFR mutation. Following hypoxic growth conditions, HCC827 cells treated with gefitinib upregulated N-cadherin, fibronectin, and vimentin expression and downregulated E-cadherin, characteristic of an epithelial-mesenchymal transition (EMT), which prior studies have linked to EGFR TKI resistance. Mechanistically, knockdown of the histone demethylases, LSD1 and PLU-1, prevented and reversed hypoxia-induced gefitinib resistance, with inhibition of the associated EMT, suggesting that LSD1 and PLU-1 play key roles in hypoxia-induced gefitinib resistance and EMT. Moreover, hypoxia-treated HCC827 cells demonstrated more aggressive tumor growth in vivo compared with cells grown in normoxia, but inhibition of LSD1 function by shRNA-mediated knockdown or by the small-molecular inhibitor SP2509 suppressed tumor growth and enhanced gefitinib response in vivo These results suggest that hypoxia is a driving force for acquired resistance to EGFR TKIs through epigenetic change and coordination of EMT in NSCLC. This study suggests that combination of therapy with EGFR TKIs and LSD1 inhibitors may offer an attractive therapeutic strategy for NSCLCs. Mol Cancer Res; 16(10); 1458-69. (c)2018 AACR.
Stress-induced phosphoprotein 1 acts as a scaffold protein for glycogen synthase kinase-3 beta-mediated phosphorylation of lysine-specific demethylase 1.[Pubmed:29593255]
Oncogenesis. 2018 Mar 29;7(3):31.
Stress-induced phosphoprotein 1 (STIP1)-a co-chaperone of heat shock proteins-promotes cell proliferation and may act as an oncogenic factor. Similarly, glycogen synthase kinase-3 beta (GSK3beta)-mediated phosphorylation of lysine-specific demethylase 1 (LSD1)-an epigenetic regulator-can contribute to the development of an aggressive cell phenotype. Owing to their ability to tether different molecules into functional complexes, scaffold proteins have a key role in the regulation of different signaling pathways in tumorigenesis. Here, we show that STIP1 acts as a scaffold promoting the interaction between LSD1 and GSK3beta. Specifically, the TPR1 and TPR2B domains of STIP1 are capable of binding with the AOL domain of LSD1, whereas the TPR2A and TPR2B domains of STIP1 interact with the kinase domain of GSK3beta. We also demonstrate that STIP1 is required for GSK3beta-mediated LSD1 phosphorylation, which promoted LSD1 stability and enhanced cell proliferation. After transfection of cancer cells with double-mutant (S707A/S711A) LSD1, subcellular localization analysis revealed that LSD1 was translocated from the nucleus to the cytoplasm. In vitro experiments also showed that the LSD1 inhibitor SP2509 and the GSK3beta inhibitor LY2090314 acted synergistically to induce cancer cell death. Finally, the immunohistochemical expression of STIP1 and LSD1 showed a positively correlation in human cancer specimens. In summary, our data provide mechanistic insights into the role of STIP1 in human tumorigenesis by showing that it serves as a scaffold for GSK3beta-mediated LSD1 phosphorylation. The combination of LSD1 and GSK3beta inhibitors may exert synergistic antitumor effects and deserves further scrutiny in preclinical studies.
Loss of NR2E3 represses AHR by LSD1 reprogramming, is associated with poor prognosis in liver cancer.[Pubmed:28878246]
Sci Rep. 2017 Sep 6;7(1):10662.
The aryl hydrocarbon receptor (AHR) plays crucial roles in inflammation, metabolic disorder, and cancer. However, the molecular mechanisms regulating AHR expression remain unknown. Here, we found that an orphan nuclear NR2E3 maintains AHR expression, and forms an active transcriptional complex with transcription factor Sp1 and coactivator GRIP1 in MCF-7 human breast and HepG2 liver cancer cell lines. NR2E3 loss promotes the recruitment of LSD1, a histone demethylase of histone 3 lysine 4 di-methylation (H3K4me2), to the AHR gene promoter region, resulting in repression of AHR expression. AHR expression and responsiveness along with H3K4me2 were significantly reduced in the livers of Nr2e3(rd7) (Rd7) mice that express low NR2E3 relative to the livers of wild-type mice. SP2509, an LSD1 inhibitor, fully restored AHR expression and H3K4me2 levels in Rd7 mice. Lastly, we demonstrated that both AHR and NR2E3 are significantly associated with good clinical outcomes in liver cancer. Together, our results reveal a novel link between NR2E3, AHR, and liver cancer via LSD1-mediated H3K4me2 histone modification in liver cancer development.
Lysine-specific demethylase LSD1 regulates autophagy in neuroblastoma through SESN2-dependent pathway.[Pubmed:28783174]
Oncogene. 2017 Nov 30;36(48):6701-6711.
Autophagy is a physiological process, important for recycling of macromolecules and maintenance of cellular homeostasis. Defective autophagy is associated with tumorigenesis and has a causative role in chemotherapy resistance in leukemia and in solid cancers. Here, we report that autophagy is regulated by the lysine-specific demethylase LSD1/KDM1A, an epigenetic marker whose overexpression is a feature of malignant neoplasia with an instrumental role in cancer development. In the present study, we determine that two different LSD1 inhibitors (TCP and SP2509) as well as selective ablation of LSD1 expression promote autophagy in neuroblastoma cells. At a mechanistic level, we show that LSD1 binds to the promoter region of Sestrin2 (SESN2), a critical regulator of mTORC1 activity. Pharmacological inhibition of LSD1 triggers SESN2 expression that hampers mTORC1 activity, leading to enhanced autophagy. SESN2 overexpression suffices to promote autophagy in neuroblastoma cells, while loss of SESN2 expression reduces autophagy induced by LSD1 inhibition. Our findings elucidate a mechanism whereby LSD1 controls autophagy in neuroblastoma cells through SESN2 transcription regulation, and we suggest that pharmacological targeting of LSD1 may have effective therapeutic relevance in the control of autophagy in neuroblastoma.
Stepwise assembly of functional C-terminal REST/NRSF transcriptional repressor complexes as a drug target.[Pubmed:28218430]
Protein Sci. 2017 May;26(5):997-1011.
In human cells, thousands of predominantly neuronal genes are regulated by the repressor element 1 (RE1)-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF). REST/NRSF represses transcription of these genes in stem cells and non-neuronal cells by tethering corepressor complexes. Aberrant REST/NRSF expression and intracellular localization are associated with cancer and neurodegeneration in humans. To date, detailed molecular analyses of REST/NRSF and its C-terminal repressor complex have been hampered largely by the lack of sufficient amounts of purified REST/NRSF and its complexes. Therefore, the aim of this study was to express and purify human REST/NRSF and its C-terminal interactors in a baculovirus multiprotein expression system as individual proteins and coexpressed complexes. All proteins were enriched in the nucleus, and REST/NRSF was isolated as a slower migrating form, characteristic of nuclear REST/NRSF in mammalian cells. Both REST/NRSF alone and its C-terminal repressor complex were functionally active in histone deacetylation and histone demethylation and bound to RE1/neuron-restrictive silencer element (NRSE) sites. Additionally, the mechanisms of inhibition of the small-molecule drugs 4SC-202 and SP2509 were analyzed. These drugs interfered with the viability of medulloblastoma cells, where REST/NRSF has been implicated in cancer pathogenesis. Thus, a resource for molecular REST/NRSF studies and drug development has been established.
Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells.[Pubmed:24699304]
Leukemia. 2014 Nov;28(11):2155-64.
The histone demethylase LSD1 (KDM1A) demethylates mono- and di-methylated (Me2) lysine (K) 4 on histone H3. High LSD1 expression blocks differentiation and confers a poor prognosis in acute myeloid leukemia (AML). Here, treatment with the novel LSD1 antagonist SP2509 attenuated the binding of LSD1 with the corepressor CoREST, increased the permissive H3K4Me3 mark on the target gene promoters, and increased the levels of p21, p27 and CCAAT/enhancer binding protein alpha in cultured AML cells. In addition, SP2509 treatment or LSD1 shRNA inhibited the colony growth of AML cells. SP2509 also induced morphological features of differentiation in the cultured and primary AML blasts. SP2509 induced more apoptosis of AML cells expressing mutant NPM1 than mixed-lineage leukemia fusion oncoproteins. Treatment with SP2509 alone significantly improved the survival of immune-depleted mice following tail-vein infusion and engraftment of cultured or primary human AML cells. Co-treatment with pan-HDAC inhibitor (HDI) panobinostat (PS) and SP2509 was synergistically lethal against cultured and primary AML blasts. Compared with each agent alone, co-treatment with SP2509 and PS significantly improved the survival of the mice engrafted with the human AML cells, without exhibiting any toxicity. Collectively, these findings show that the combination of LSD1 antagonist and pan-HDI is a promising therapy warranting further testing against AML.


