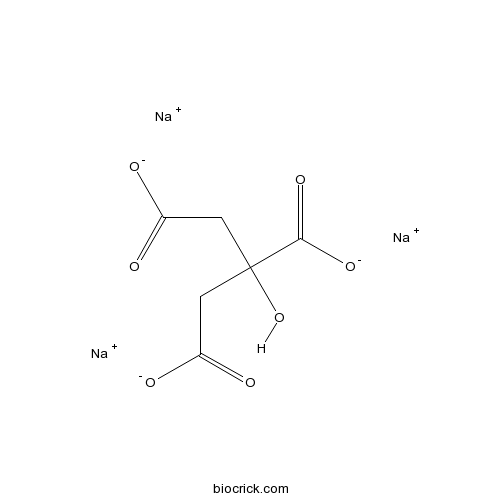
Sodium citrateCAS# 68-04-2 |
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68-04-2 | SDF | Download SDF |
| PubChem ID | 6224 | Appearance | Powder |
| Formula | C6H5Na3O7 | M.Wt | 258.07 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 3000 mM in water | ||
| Chemical Name | 2-Hydroxy-1,2,3-propanenetricarboxy | ||
| SMILES | C(C(=O)[O-])C(CC(=O)[O-])(C(=O)[O-])O.[Na+].[Na+].[Na+] | ||
| Standard InChIKey | HRXKRNGNAMMEHJ-UHFFFAOYSA-K | ||
| Standard InChI | InChI=1S/C6H8O7.3Na/c7-3(8)1-6(13,5(11)12)2-4(9)10;;;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);;;/q;3*+1/p-3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Commonly used laboratory reagent |

Sodium citrate Dilution Calculator

Sodium citrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8749 mL | 19.3746 mL | 38.7492 mL | 77.4984 mL | 96.8729 mL |
| 5 mM | 0.775 mL | 3.8749 mL | 7.7498 mL | 15.4997 mL | 19.3746 mL |
| 10 mM | 0.3875 mL | 1.9375 mL | 3.8749 mL | 7.7498 mL | 9.6873 mL |
| 50 mM | 0.0775 mL | 0.3875 mL | 0.775 mL | 1.55 mL | 1.9375 mL |
| 100 mM | 0.0387 mL | 0.1937 mL | 0.3875 mL | 0.775 mL | 0.9687 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BMS 193885
Catalog No.:BCC7613
CAS No.:679839-66-8
- N-(15-Methyl-9-hexadecenoyl)taurine
Catalog No.:BCN1754
CAS No.:679834-30-1
- N-(5,8,11,14-Eicosatetraenoyl)taurine
Catalog No.:BCN1755
CAS No.:679834-28-7
- Aurantio-obtusin
Catalog No.:BCN1222
CAS No.:67979-25-3
- Sodium Danshensu
Catalog No.:BCN5952
CAS No.:67920-52-9
- 1,6-Dihydro-4,7'-epoxy-1-methoxy-3',4'-methylenedioxy-6-oxo-3,8'-lignan
Catalog No.:BCN6584
CAS No.:67920-48-3
- Mesaconine
Catalog No.:BCC8339
CAS No.:6792-09-2
- Macusine B
Catalog No.:BCN6471
CAS No.:6792-07-0
- Homobaldrinal
Catalog No.:BCN2681
CAS No.:67910-07-0
- Dehydroevodiamine
Catalog No.:BCN2974
CAS No.:67909-49-3
- Aluminum n-octacosoxide
Catalog No.:BCC8099
CAS No.:67905-27-5
- Ambrox
Catalog No.:BCN6907
CAS No.:6790-58-5
- Vitamin B12
Catalog No.:BCC4878
CAS No.:68-19-9
- Norethindrone
Catalog No.:BCC4811
CAS No.:68-22-4
- Vitamin A
Catalog No.:BCN8349
CAS No.:68-26-8
- Sulfadiazine
Catalog No.:BCC3859
CAS No.:68-35-9
- Hydroxyzine
Catalog No.:BCC5209
CAS No.:68-88-2
- Metamizole sodium
Catalog No.:BCC9024
CAS No.:68-89-3
- Hypoxanthine
Catalog No.:BCC5324
CAS No.:68-94-0
- Ac-Pro-OH
Catalog No.:BCC3017
CAS No.:68-95-1
- Hydroxyprogesterone
Catalog No.:BCC8996
CAS No.:68-96-2
- Magnocurarine
Catalog No.:BCN3839
CAS No.:6801-40-7
- Pulsatilla saponin H
Catalog No.:BCN8181
CAS No.:68027-14-5
- Pulsatilla saponin D
Catalog No.:BCN8526
CAS No.:68027-15-6
A randomised controlled trial of sodium citrate spray for non-conductive olfactory disorders.[Pubmed:28339165]
Clin Otolaryngol. 2017 Dec;42(6):1295-1302.
OBJECTIVES: Previous research has suggested that Sodium citrate improves hyposmia by decreasing mucus calcium levels in the nose. This study aimed to confirm or refute this effect in a single application and assess potential side-effects. DESIGN: Study design was a randomised double-blind controlled trial of Sodium citrate nasal spray (intervention) vs sterile water (control). Fifty-five patients with non-conductive olfactory loss were randomised to receive the intervention or placebo. SETTING: Tertiary care clinic. MAIN OUTCOME MEASURES: The primary outcome measure was improvement in measured olfactory thresholds for phenyl ethyl alcohol (PEA) over 2 hours. Other outcome measures assessed were improvement in olfactory thresholds in 1-butanol, eucalyptol and acetic acid; number of responders with a clinically relevant response in each arm; and adverse effects. RESULTS: A significant effect was seen in the intervention arm for PEA and for 1-butanol and eucalyptol when compared to the control arm (P<.05); 32% of the intervention arm responded in terms of improved sensitivity towards some of the odours. Minor adverse effects noted included sore throat, nasal paraesthesia, slight rhinorrhoea and itching. The duration of effect of the citrate is transient, peaking at 30-60 minutes after application. CONCLUSIONS: Sodium citrate yields some potential as a treatment for non-conductive olfactory loss; however, these findings require corroboration in further clinical trials looking at longer term regular use of the spray as a viable therapeutic option for patients where it would be applied at frequent intervals such as before mealtimes.
Ethylenediaminetetraacetic Acid, Sodium Citrate, Heparin and Citrate Phosphate Dextrose-Adenine Anticoagulants Differentially Affect Cytokine mRNA Expression in Blood Leukocytes.[Pubmed:28164647]
Clin Lab. 2016 Jul 1;62(7):1371-1374.
BACKGROUND: The aim of this study is to determine whether and how the anticoagulant agents EDTA, Sodium citrate, and heparin present in vacutainers and CPDA located in transfusion bags modify peripheral blood cell behavior. METHODS: We compared the effect of four anticoagulants within an expression study quantifying mRNA by realtime PCR in 60 blood samples. RESULTS: We observed a significant increase of TNFalpha and CCL2 mRNA expression in leukocytes placed in EDTA anticoagulant compared to leukocytes collected with citrate or heparin (p


