SulfadiazineCAS# 68-35-9 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68-35-9 | SDF | Download SDF |
| PubChem ID | 5215 | Appearance | Powder |
| Formula | C10H10N4O2S | M.Wt | 250.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (199.78 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
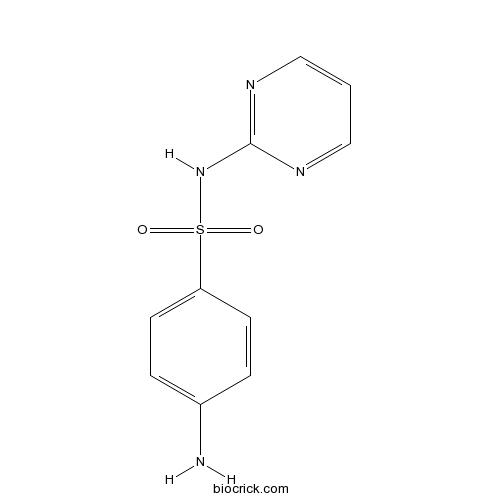
| Chemical Name | 4-amino-N-pyrimidin-2-ylbenzenesulfonamide | ||
| SMILES | C1=CN=C(N=C1)NS(=O)(=O)C2=CC=C(C=C2)N | ||
| Standard InChIKey | SEEPANYCNGTZFQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H10N4O2S/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10/h1-7H,11H2,(H,12,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sulfadiazine is a sulfonamide antibiotic.
Target: Antibacterial
Sulfadiazine eliminates bacteria that cause infections by stopping the production of folate inside the bacterial cell, and is commonly used to treat urinary tract infections (UTIs). In combination, sulfadiazine and pyrimethamine, can be used to treat toxoplasmosis, a disease caused by Toxoplasma gondii. From Wikipedia.
The ulcers who treated with silver sulfadiazine cream responded rapidly, with one-third showing bacterial levels of less than 10(5) within three days, and half within a week [1]. References: | |||||

Sulfadiazine Dilution Calculator

Sulfadiazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9955 mL | 19.9776 mL | 39.9553 mL | 79.9105 mL | 99.8881 mL |
| 5 mM | 0.7991 mL | 3.9955 mL | 7.9911 mL | 15.9821 mL | 19.9776 mL |
| 10 mM | 0.3996 mL | 1.9978 mL | 3.9955 mL | 7.9911 mL | 9.9888 mL |
| 50 mM | 0.0799 mL | 0.3996 mL | 0.7991 mL | 1.5982 mL | 1.9978 mL |
| 100 mM | 0.04 mL | 0.1998 mL | 0.3996 mL | 0.7991 mL | 0.9989 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
An antibacterial.
- Vitamin A
Catalog No.:BCN8349
CAS No.:68-26-8
- Norethindrone
Catalog No.:BCC4811
CAS No.:68-22-4
- Vitamin B12
Catalog No.:BCC4878
CAS No.:68-19-9
- Sodium citrate
Catalog No.:BCC7588
CAS No.:68-04-2
- BMS 193885
Catalog No.:BCC7613
CAS No.:679839-66-8
- N-(15-Methyl-9-hexadecenoyl)taurine
Catalog No.:BCN1754
CAS No.:679834-30-1
- N-(5,8,11,14-Eicosatetraenoyl)taurine
Catalog No.:BCN1755
CAS No.:679834-28-7
- Aurantio-obtusin
Catalog No.:BCN1222
CAS No.:67979-25-3
- Sodium Danshensu
Catalog No.:BCN5952
CAS No.:67920-52-9
- 1,6-Dihydro-4,7'-epoxy-1-methoxy-3',4'-methylenedioxy-6-oxo-3,8'-lignan
Catalog No.:BCN6584
CAS No.:67920-48-3
- Mesaconine
Catalog No.:BCC8339
CAS No.:6792-09-2
- Macusine B
Catalog No.:BCN6471
CAS No.:6792-07-0
- Hydroxyzine
Catalog No.:BCC5209
CAS No.:68-88-2
- Metamizole sodium
Catalog No.:BCC9024
CAS No.:68-89-3
- Hypoxanthine
Catalog No.:BCC5324
CAS No.:68-94-0
- Ac-Pro-OH
Catalog No.:BCC3017
CAS No.:68-95-1
- Hydroxyprogesterone
Catalog No.:BCC8996
CAS No.:68-96-2
- Magnocurarine
Catalog No.:BCN3839
CAS No.:6801-40-7
- Pulsatilla saponin H
Catalog No.:BCN8181
CAS No.:68027-14-5
- Pulsatilla saponin D
Catalog No.:BCN8526
CAS No.:68027-15-6
- Carbadox
Catalog No.:BCC3744
CAS No.:6804-07-5
- (Z)-4-Hydroxytamoxifen
Catalog No.:BCC6015
CAS No.:68047-06-3
- Escin
Catalog No.:BCC8323
CAS No.:6805-41-0
- Platyconic acid A
Catalog No.:BCN3239
CAS No.:68051-23-0
Technetium-99m-Labeled Sulfadiazine: a Targeting Radiopharmaceutical for Scintigraphic Imaging of Infectious Foci Due To Escherichia coli in Mouse and Rabbit Models.[Pubmed:28285355]
Appl Biochem Biotechnol. 2017 Sep;183(1):374-384.
Bacterial infection is one of the vital reasons of morbidity and mortality, especially in developing countries. It appears silently without bothering the geological borders and imposes a grave threat to humanity. Nuclear medicine technique has an important role in helping early diagnosis of deep-seated infections. The aim of this study was to develop a new radiopharmaceutical (99m)Tc-labeling Sulfadiazine as an infection imaging agent. Radiolabeling of Sulfadiazine with technetium-99m ((99m)Tc) was carried out using stannous tartrate as a reducing agent in the presence of gentistic acid at pH = 5. The quality control tests revealed ~98% labeling efficiency. Paper chromatographic (PC) and instant thin-layer chromatographic (ITLC) techniques were used to analyze radiochemical yield. Biodistribution and infection specificity of the radiotracer were performed with Escherichia coli (E. coli) infection-induced rats. Scintigraphy and glomerular filtration rate (GFR) study was performed in E. coli-infected rabbits. Scintigraphy indicated E. coli infection targeting potential of (99m)Tc-SDZ, while biodistribution study showed minimal uptake of (99m)Tc-SDZ in non-targeted tissues. The uptake in the kidneys was found 2.56 +/- 0.06, 2.09 +/- 0.10, and 1.68 +/- 0.09% at 30 min, 1 h, and 4 h, respectively. The infected muscle (target) to non-infected muscle (non-target) ratio (T/NT) was found 4.49 +/- 0.04, 6.78 +/- 0.07, and 5.59 +/- 0.08 at 30 min, 1 h, and 4 h, respectively.
Heterogeneity of mast cells and expression of Annexin A1 protein in a second degree burn model with silver sulfadiazine treatment.[Pubmed:28278234]
PLoS One. 2017 Mar 9;12(3):e0173417.
Mast cells (MCs) participate in all stages of skin healing and one of their mediators is the Annexin A1 protein (AnxA1), linked to inflammation, proliferation, migration and apoptosis processes, but not studied in thermal burns yet. Therefore, our objectives were to evaluate the behavior of MCs and AnxA1 in a second degree burn model, treated or not with silver Sulfadiazine 1% (SDP 1%) and associated to macrophages quantification and cytokines dosages. MCs counts showed few cells in the early stages of repair but increased MCs in the final phases in the untreated group. The normal skin presented numerous tryptase-positive MCs that were reduced after burning in all analyzed periods. Differently, few chymase-positive MCs were observed in the early stages of healing, however, increased chymase-positive MCs were found at the final phase in the untreated group. MCs also showed high immunoreactivity for AnxA1 on day 3 in both groups. In the tissue there was a strong protein expression in the early stages of healing, but in the final phases only in the SDP treated animals. TNF-alpha, IL-1beta, IL-6, IL-10 and MCP-1 levels and macrophages quantification were increased in inflammation and reepithelialization phases. Reduced IL-1beta, IL-6 and IL-10 levels and numerous macrophages occurred in the treated animals during tissue repair. Our results indicate modulation in the profile of MCs and AnxA1expression during healing by the treatment with SDP 1%, pointing them as targets for therapeutic interventions on skin burns.
Sulfate radical-based oxidation of antibiotics sulfamethazine, sulfapyridine, sulfadiazine, sulfadimethoxine, and sulfachloropyridazine: Formation of SO2 extrusion products and effects of natural organic matter.[Pubmed:28363182]
Sci Total Environ. 2017 Sep 1;593-594:704-712.
The widespread occurrence of sulfonamide antibiotics in the environment has raised great concerns about their potential to proliferate antibacterial resistance. Sulfate radical (SO4(*-)) based advanced oxidation processes (SR-AOPs) are promising in-situ chemical oxidation (ISCO) technologies for remediation of soil and groundwater contaminated by antibiotics. The present study reported that thermally activated persulfate oxidation of sulfonamides (SAs) bearing six-membered heterocyclic rings, e.g., sulfamethazine (SMZ), sulfapyridine (SPD), Sulfadiazine (SDZ), sulfadimethoxine (SDM), and sulfachloropyridazine (SCP), all produced SO2 extrusion products (SEPs), a phenomenon that is of potential importance, but not systematically studied. As an electrophilic oxidant, SO4(*-) tends to attack the aniline moiety, the reactive site of SAs, via electro-transfer mechanism. The resulting anilinyl radical cations are subjected to further intermolecular Smiles-type rearrangement to produce SEPs. Formation of SEPs is expected to occur in other SR-AOPs as well. The temperature-dependent evolution pattern of SEP of SMZ, 4-(2-imino-4,6-dimethylpyrimidin-1(2H)-yl)aniline, can be well fitted by kinetic modeling concerning sequential formation and transformation of intermediate product. The presence of natural organic matter (NOM) influenced the evolution patterns of 4-(2-imino-4,6-dimethylpyrimidin-1(2H)-yl)aniline significantly. Toxicological effects of SEPs on ecosystem and human health remain largely unknown, thus, further monitoring studies are highly desirable.
Impact of sulfadiazine on performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater.[Pubmed:28365339]
Bioresour Technol. 2017 Jul;235:122-130.
The impact of Sulfadiazine on the performance, microbial activity and microbial community of a sequencing batch biofilm reactor (SBBR) were evaluated in treating mariculture wastewater due to the application of Sulfadiazine as an antibiotic in mariculture. The COD and nitrogen removals kept stable at 0-6mg/L Sulfadiazine and were inhibited at 10-35mg/L Sulfadiazine. The microbial activities related to organic matter and nitrogen removals reduced with an increase in Sulfadiazine concentration. The presence of Sulfadiazine could affect the production and chemical composition of loosely bound extracellular polymeric substances (LB-EPS) and tightly bound EPS (TB-EPS) in the biofilm. High-throughput sequencing demonstrated that Sulfadiazine could impact on the microbial richness and diversity of SBBR treating mariculture wastewater. The relative abundances of Nitrosomonas, Nitrospira, Paracoccus, Hyphomicrobium, Rhodanobacter, Thauera and Steroidobacter decreased with an increase in Sulfadiazine concentration, indicating that the presence of Sulfadiazine decreased the relative abundance of some nitrifying and denitrifying bacteria.


