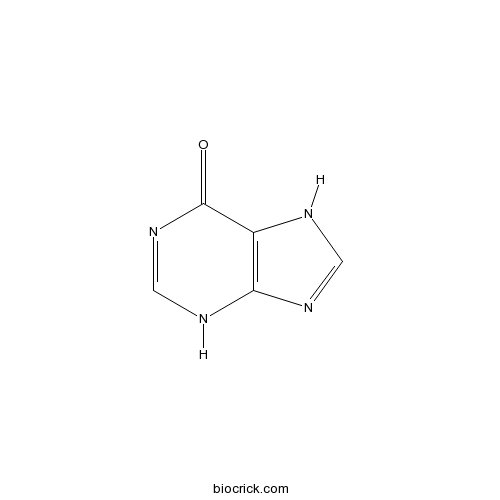
HypoxanthineCAS# 68-94-0 |
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68-94-0 | SDF | Download SDF |
| PubChem ID | 790 | Appearance | Powder |
| Formula | C5H4N4O | M.Wt | 136.11 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 10 mg/mL (73.47 mM; Need ultrasonic) | ||
| Chemical Name | 3,7-dihydropurin-6-one | ||
| SMILES | C1=NC2=C(N1)C(=O)N=CN2 | ||
| Standard InChIKey | FDGQSTZJBFJUBT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H4N4O/c10-5-3-4(7-1-6-3)8-2-9-5/h1-2H,(H2,6,7,8,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hypoxanthine, a purine derivative, is a potential free radical generator and could be used as an indicator of hypoxia.Hypoxanthine inhibits mouse oocyte maturation, it plays a role in vivo in maintaining meiotic arrest. |
| In vitro | The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin-induced oocyte maturation.[Pubmed: 3100361]Dev Biol. 1987 Feb;119(2):313-21.The concentration of Hypoxanthine in mouse follicular fluid has been estimated to be 2-4 mM, and although this concentration maintains meiotic arrest in fully grown mouse oocytes in vitro, oocyte maturation in vivo is not induced by a decrease in the concentration of this purine in follicular fluid (J. J. Eppig, P. F. Ward-Bailey, and D. L. Coleman, Biol. Reprod. 33, 1041-1049, 1985). |
| In vivo | Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low molecular weight fraction of porcine follicular fluid.[Reference: WebLink]Proceedings of the National Academy of Sciences of the United States of America, 1985, 82(2):454-8.Studies were carried out to identify and quantify the porcine follicular fluid (PFF) component(s) responsible for inhibition of murine oocyte maturation. |
| Kinase Assay | Hypoxanthine as an Indicator of Hypoxia: Its Role in Health and Disease through Free Radical Production[Reference: WebLink]Pediatric Research, 1988, 23(2):143-50.Hypoxia is a common insult during the perinatal and neonatal period. New a n d better ways t o evaluate hypoxia are needed. |

Hypoxanthine Dilution Calculator

Hypoxanthine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.347 mL | 36.735 mL | 73.47 mL | 146.94 mL | 183.675 mL |
| 5 mM | 1.4694 mL | 7.347 mL | 14.694 mL | 29.388 mL | 36.735 mL |
| 10 mM | 0.7347 mL | 3.6735 mL | 7.347 mL | 14.694 mL | 18.3675 mL |
| 50 mM | 0.1469 mL | 0.7347 mL | 1.4694 mL | 2.9388 mL | 3.6735 mL |
| 100 mM | 0.0735 mL | 0.3673 mL | 0.7347 mL | 1.4694 mL | 1.8367 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Metamizole sodium
Catalog No.:BCC9024
CAS No.:68-89-3
- Hydroxyzine
Catalog No.:BCC5209
CAS No.:68-88-2
- Sulfadiazine
Catalog No.:BCC3859
CAS No.:68-35-9
- Vitamin A
Catalog No.:BCN8349
CAS No.:68-26-8
- Norethindrone
Catalog No.:BCC4811
CAS No.:68-22-4
- Vitamin B12
Catalog No.:BCC4878
CAS No.:68-19-9
- Sodium citrate
Catalog No.:BCC7588
CAS No.:68-04-2
- BMS 193885
Catalog No.:BCC7613
CAS No.:679839-66-8
- N-(15-Methyl-9-hexadecenoyl)taurine
Catalog No.:BCN1754
CAS No.:679834-30-1
- N-(5,8,11,14-Eicosatetraenoyl)taurine
Catalog No.:BCN1755
CAS No.:679834-28-7
- Aurantio-obtusin
Catalog No.:BCN1222
CAS No.:67979-25-3
- Sodium Danshensu
Catalog No.:BCN5952
CAS No.:67920-52-9
- Ac-Pro-OH
Catalog No.:BCC3017
CAS No.:68-95-1
- Hydroxyprogesterone
Catalog No.:BCC8996
CAS No.:68-96-2
- Magnocurarine
Catalog No.:BCN3839
CAS No.:6801-40-7
- Pulsatilla saponin H
Catalog No.:BCN8181
CAS No.:68027-14-5
- Pulsatilla saponin D
Catalog No.:BCN8526
CAS No.:68027-15-6
- Carbadox
Catalog No.:BCC3744
CAS No.:6804-07-5
- (Z)-4-Hydroxytamoxifen
Catalog No.:BCC6015
CAS No.:68047-06-3
- Escin
Catalog No.:BCC8323
CAS No.:6805-41-0
- Platyconic acid A
Catalog No.:BCN3239
CAS No.:68051-23-0
- EMPA
Catalog No.:BCC6226
CAS No.:680590-49-2
- Megastigm-7-ene-3,5,6,9-tetraol
Catalog No.:BCN5169
CAS No.:680617-50-9
- Trifolirhizin
Catalog No.:BCN4237
CAS No.:6807-83-6
The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin-induced oocyte maturation.[Pubmed:3100361]
Dev Biol. 1987 Feb;119(2):313-21.
The concentration of Hypoxanthine in mouse follicular fluid has been estimated to be 2-4 mM, and although this concentration maintains meiotic arrest in fully grown mouse oocytes in vitro, oocyte maturation in vivo is not induced by a decrease in the concentration of this purine in follicular fluid (J. J. Eppig, P. F. Ward-Bailey, and D. L. Coleman, Biol. Reprod. 33, 1041-1049, 1985). In the present study, the effect of 2 mM Hypoxanthine on oocyte growth and development in vitro was assessed and the ability of gonadotropins to stimulate oocyte maturation in the continued presence of Hypoxanthine was determined. Oocyte-granulosa cell complexes were isolated from 10- to 11-day-old mice and cultured in the presence or absence of 2 mM Hypoxanthine. Oocytes from 10- to 11-day-old mice are in mid-growth phase and, without further development, are incompetent of undergoing meiotic maturation. During a 12-day culture period the granulosa cell-enclosed oocytes approximately doubled in size and, regardless of the presence or absence of Hypoxanthine, 50-70% developed competence to undergo germinal vesicle breakdown (GVBD). Hypoxanthine promoted the continued association of oocytes with their companion granulosa cells during the 12-day culture period, and therefore had a beneficial effect on oocyte development. Most of the oocytes that acquired GVBD competence in the absence of Hypoxanthine underwent spontaneous GVBD. In contrast, 95% of the GVBD-competent oocytes were maintained in meiotic arrest by Hypoxanthine. Following withdrawal of the Hypoxanthine after the 12-day culture, 75% of the GVBD-competent oocytes underwent GVBD. These results show that Hypoxanthine, and/or its metabolites, maintains meiotic arrest in oocytes that grow and acquire GVBD competence in vitro. Follicle-stimulating hormone (FSH), but not luteinizing hormone or human chorionic gonadotropin, induced oocyte GVBD in the continued presence of Hypoxanthine. FSH stimulated oocyte maturation at a significantly (P less than 0.01) higher frequency than coculture of the granulosa cell-denuded oocytes with granulosa cells in the continued presence of Hypoxanthine. FSH did not induce the maturation of denuded oocytes cocultured with granulosa cells.(ABSTRACT TRUNCATED AT 400 WORDS)


