VcMMAEAntibody-drug conjugate CAS# 646502-53-6 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 646502-53-6 | SDF | Download SDF |
| PubChem ID | 46944733 | Appearance | Powder |
| Formula | C68H105N11O15 | M.Wt | 1316.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | mc-vc-PAB-MMAE | ||
| Solubility | DMSO : ≥ 54 mg/mL (41.01 mM) *"≥" means soluble, but saturation unknown. | ||
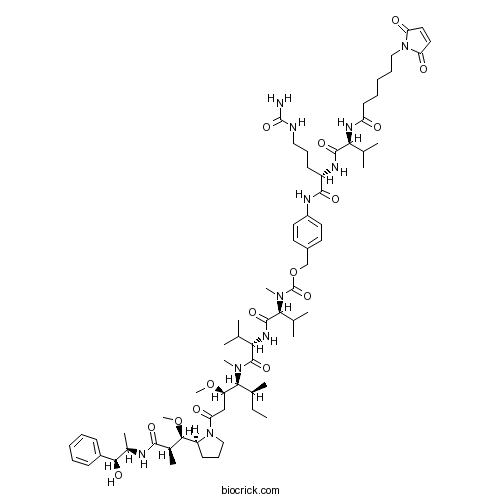
| Chemical Name | [4-[[(2S)-5-(carbamoylamino)-2-[[(2S)-2-[6-(2,5-dioxopyrrol-1-yl)hexanoylamino]-3-methylbutanoyl]amino]pentanoyl]amino]phenyl]methyl N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylcarbamate | ||
| SMILES | CCC(C)C(C(CC(=O)N1CCCC1C(C(C)C(=O)NC(C)C(C2=CC=CC=C2)O)OC)OC)N(C)C(=O)C(C(C)C)NC(=O)C(C(C)C)N(C)C(=O)OCC3=CC=C(C=C3)NC(=O)C(CCCNC(=O)N)NC(=O)C(C(C)C)NC(=O)CCCCCN4C(=O)C=CC4=O | ||
| Standard InChIKey | NLMBVBUNULOTNS-HOKPPMCLSA-N | ||
| Standard InChI | InChI=1S/C68H105N11O15/c1-15-43(8)59(51(92-13)38-55(83)78-37-23-27-50(78)61(93-14)44(9)62(85)71-45(10)60(84)47-24-18-16-19-25-47)76(11)66(89)57(41(4)5)75-65(88)58(42(6)7)77(12)68(91)94-39-46-29-31-48(32-30-46)72-63(86)49(26-22-35-70-67(69)90)73-64(87)56(40(2)3)74-52(80)28-20-17-21-36-79-53(81)33-34-54(79)82/h16,18-19,24-25,29-34,40-45,49-51,56-61,84H,15,17,20-23,26-28,35-39H2,1-14H3,(H,71,85)(H,72,86)(H,73,87)(H,74,80)(H,75,88)(H3,69,70,90)/t43-,44+,45+,49-,50-,51+,56-,57-,58-,59-,60+,61+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | VcMMAE is a drug-linker conjugate for ADC with potent antitumor activity by using the anti-mitotic agent, monomethyl auristatin E (MMAE), linked via the lysosomally cleavable dipeptide, valine-citrulline (vc).In Vitro:Monomethyl auristatin E (MMAE) is efficiently released from SGN-35 within CD30+ cancer cells and, due to its membrane permeability, is able to exert cytotoxic activity on bystander cells[1]. MMAE sensitized colorectal and pancreatic cancer cells to IR in a schedule and dose dependent manner correlating with mitotic arrest. Radiosensitization is evidenced by decreased clonogenic survival and increased DNA double strand breaks in irradiated cells[2].In Vivo:Monomethyl auristatin E (MMAE) in combination with IR results in tumor growth delay, tumor-targeted ACPP-cRGD-MMAE with IR produces a more robust and significantly prolonged tumor regression in xenograft models[2]. References: | |||||

VcMMAE Dilution Calculator

VcMMAE Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.7595 mL | 3.7976 mL | 7.5951 mL | 15.1903 mL | 18.9879 mL |
| 5 mM | 0.1519 mL | 0.7595 mL | 1.519 mL | 3.0381 mL | 3.7976 mL |
| 10 mM | 0.076 mL | 0.3798 mL | 0.7595 mL | 1.519 mL | 1.8988 mL |
| 50 mM | 0.0152 mL | 0.076 mL | 0.1519 mL | 0.3038 mL | 0.3798 mL |
| 100 mM | 0.0076 mL | 0.038 mL | 0.076 mL | 0.1519 mL | 0.1899 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
VcMMAE is an antibody-drug conjugate (ADC) with potent antitumor activity by using the anti-mitotic agent, monomethyl auristatin E (MMAE), linked via the lysosomally cleavable dipeptide, valine-citrulline (vc). Antibody-drug conjugates (ADCs) are designed to combine the exquisite specificity of antibodies to target tumor antigens with the cytotoxic potency of chemotherapeutic drugs. In vivo studies demonstrated that the peptide-linked conjugates induced regressions and cures of established tumor xenografts with therapeutic indices as high as 60-fold. These conjugates illustrate the importance of linker technology, drug potency and conjugation methodology in developing safe and efficacious mAb-drug conjugates for cancer therapy.
- H-D-Glu(OMe)-OH
Catalog No.:BCC2940
CAS No.:6461-04-7
- Sinigrin monohydrate
Catalog No.:BCN2595
CAS No.:64550-88-5
- Acantrifoside E
Catalog No.:BCN6646
CAS No.:645414-25-1
- Oxprenolol hydrochloride
Catalog No.:BCC7630
CAS No.:6452-73-9
- Scoulerine
Catalog No.:BCN6623
CAS No.:6451-73-6
- Isovanillic acid
Catalog No.:BCN3376
CAS No.:645-08-9
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Rubianthraquinone
Catalog No.:BCN6880
CAS No.:644967-44-2
- Cefotaxime sodium
Catalog No.:BCC8908
CAS No.:64485-93-4
- Glycoursodeoxycholic acid
Catalog No.:BCN7369
CAS No.:64480-66-6
- Picroside III
Catalog No.:BCN6324
CAS No.:64461-95-6
- Tizanidine HCl
Catalog No.:BCC4357
CAS No.:64461-82-1
- Demethylluvangetin
Catalog No.:BCN7569
CAS No.:64652-10-4
- 1,9-Dideoxyforskolin
Catalog No.:BCC6352
CAS No.:64657-18-7
- Coleonol B
Catalog No.:BCN4188
CAS No.:64657-21-2
- Demethylwedelolactone
Catalog No.:BCN2663
CAS No.:6468-55-9
- Dihydroflavokawain C
Catalog No.:BCC9229
CAS No.:
- Cinaciguat hydrochloride
Catalog No.:BCC8096
CAS No.:646995-35-9
- Morin dihydrate
Catalog No.:BCN8156
CAS No.:6472-38-4
- RA-V
Catalog No.:BCN3513
CAS No.:64725-24-2
- Tetrahydroalstonine
Catalog No.:BCN4189
CAS No.:6474-90-4
- 1,2,3,6,7-Pentamethoxyxanthone
Catalog No.:BCN7525
CAS No.:64756-86-1
- 6-Hydroxy-1,2,3,7-tetramethoxyxanthone
Catalog No.:BCN7565
CAS No.:64756-87-2
- L-trans-2,4-PDC
Catalog No.:BCC6595
CAS No.:64769-66-0
Pharmacologically enhanced expression of GPNMB increases the sensitivity of melanoma cells to the CR011-vcMMAE antibody-drug conjugate.[Pubmed:19383330]
Mol Oncol. 2008 Jun;2(1):81-93.
GPNMB is a melanoma-associated glycoprotein that is targeted by the CR011-VcMMAE antibody-drug conjugate (ADC). Previous studies have shown that CR011-VcMMAE induces the apoptosis of GPNMB-expressing tumor cells in vitro and tumor regression in xenograft models. This ADC is currently in clinical trials for melanoma. In the present investigation, a variety of compounds were examined for their ability to increase the expression of GPNMB by cancer cells. These experiments lead to the identification of three distinct groups of compounds that increased GPNMB, some of which were shown to enhance the sensitivity of melanoma cells to CR011-VcMMAE. These data indicate that it may be possible to increase the anticancer activity of CR011-VcMMAE through pharmacological enhancement of GPNMB expression for potential therapeutic benefit.
The HB22.7-vcMMAE antibody-drug conjugate has efficacy against non-Hodgkin lymphoma mouse xenografts with minimal systemic toxicity.[Pubmed:27506529]
Cancer Immunol Immunother. 2016 Oct;65(10):1169-75.
In this study, HB22.7, an anti-CD22 monoclonal antibody, was used for specific, targeted delivery of monomethyl auristatin E (MMAE) to non-Hodgkin lymphoma (NHL). MMAE was covalently coupled to HB22.7 through a valine-citrulline peptide linker (vc). Maleimide-functionalized VcMMAE (mal-VcMMAE) was reacted with thiols of the partially reduced mAb. Approximately 4 molecules of MMAE were conjugated to HB22.7 as determined by residual thiol measurement and hydrophobic interaction chromatography-HPLC (HIC-HPLC). HB22.7-VcMMAE antibody-drug conjugate (ADC) retained its binding to Ramos NHL cells and also exhibited potent and specific in vitro cytotoxicity on a panel of B cell NHL cell lines with IC50s of 20-284 ng/ml. HB22.7-VcMMAE also showed potent efficacy in vivo against established NHL xenografts using the DoHH2 and Granta 519 cell lines. One dose of the ADC induced complete and persistent response in all DoHH2 xenografts and 90 % of Granta xenografts. Minimal toxicity was observed. In summary, HB22.7-VcMMAE is an effective ADC that should be evaluated for clinical translation.
Cathepsin B Cleavage of vcMMAE-Based Antibody-Drug Conjugate Is Not Drug Location or Monoclonal Antibody Carrier Specific.[Pubmed:26914498]
Bioconjug Chem. 2016 Apr 20;27(4):1040-9.
Antibody-drug conjugates (ADCs) require thorough characterization and understanding of product quality attributes. The framework of many ADCs comprises one molecule of antibody that is usually conjugated with multiple drug molecules at various locations. It is unknown whether the drug release rate from the ADC is dependent on drug location, and/or local environment, dictated by the sequence and structure of the antibody carrier. This study addresses these issues with valine-citrulline-monomethylauristatin E (vc-MMAE)-based ADC molecules conjugated at reduced disulfide bonds, by evaluating the cathepsin B catalyzed drug release rate of ADC molecules with different drug distributions or antibody carriers. MMAE drug release rates at different locations on ADC I were compared to evaluate the impact of drug location. No difference in rates was observed for drug released from the V(H), V(L), or C(H)2 domains of ADC I. Furthermore, four vc-MMAE ADC molecules were chosen as substrates for cathepsin B for evaluation of Michaelis-Menten parameters. There was no significant difference in K(M) or k(cat) values, suggesting that different sequences of the antibody carrier do not result in different drug release rates. Comparison between ADCs and small molecules containing vc-MMAE moieties as substrates for cathepsin B suggests that the presence of IgG1 antibody carrier, regardless of its bulkiness, does not impact drug release rate. Finally, a molecular dynamics simulation on ADC II revealed that the val-cit moiety at each of the eight possible conjugation sites was, on average, solvent accessible over 50% of its maximum solvent accessible surface area (SASA) during a 500 ns trajectory. Combined, these results suggest that the cathepsin cleavage sites for conjugated drugs are exposed enough for the enzyme to access and that the drug release rate is rather independent of drug location or monoclonal antibody carrier. Therefore, the distribution of drug conjugation at different sites is not a critical parameter to control in manufacturing of the vc-MMAE-based ADC conjugated at reduced disulfide bonds.
Management of metastatic breast cancer with second-generation antibody-drug conjugates: focus on glembatumumab vedotin (CDX-011, CR011-vcMMAE).[Pubmed:24496926]
BioDrugs. 2014 Jun;28(3):253-63.
Exploiting the highly targeted nature of monoclonal antibodies to deliver selectively to tumor cells a cytotoxic payload is an attractive concept and the successful precedents of the recent past set the stage for broader applications in the future. Antibody-drug conjugates may currently hold an unprecedented potential; however, there are multiple unique challenges in their development, and the recent successes have come hand in hand with significant technologic advances in their chemistry and manufacturing. Over the years, multiple factors have been identified to affect the pharmacokinetic and pharmacodynamic properties of an antibody-drug conjugate, but many important details remain to be further investigated. These factors pertain to the target antigen, antibody, conjugate, linker, as well as the nature of the malignancy under treatment. Glembatumumab vedotin is an antibody-drug conjugate targeting glycoprotein non-metastatic B (GPNMB) expressed in multiple malignancies, including breast cancer. The expression of this protein has been associated with an aggressive malignant phenotype, invasive growth, angiogenesis, and generation of skeletal metastases. Glembatumumab vedotin is currently in early stages of clinical development in melanoma and breast cancer. Although in unselected patients with metastatic breast cancer glembatumumab vedotin was not superior to other agents, by virtue of its target being frequently and highly expressed in triple-negative breast cancer, its activity was particularly promising in this subset of patients. Results from the clinical studies in breast cancer as well as companion studies in melanoma indicate that a biomarker-informed approach is the optimal pathway for the future development of this drug.


