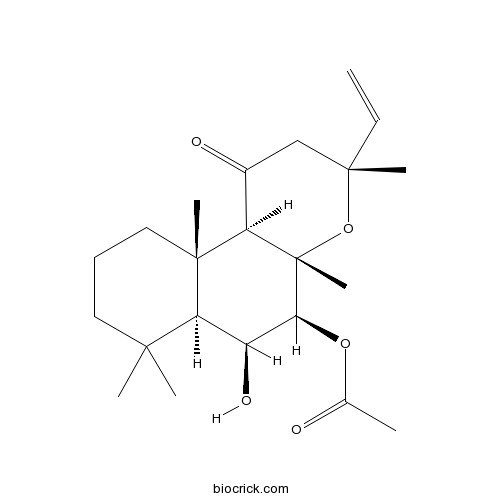
1,9-DideoxyforskolinCAS# 64657-18-7 |
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 64657-18-7 | SDF | Download SDF |
| PubChem ID | 107948 | Appearance | Powder |
| Formula | C22H34O5 | M.Wt | 378.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in DMSO | ||
| Chemical Name | [(3R,4aS,5S,6S,6aS,10aS,10bR)-3-ethenyl-6-hydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-2,5,6,6a,8,9,10,10b-octahydrobenzo[f]chromen-5-yl] acetate | ||
| SMILES | CC(=O)OC1C(C2C(CCCC2(C3C1(OC(CC3=O)(C)C=C)C)C)(C)C)O | ||
| Standard InChIKey | ZKZMDXUDDJYAIB-SUCLLAFCSA-N | ||
| Standard InChI | InChI=1S/C22H34O5/c1-8-20(5)12-14(24)16-21(6)11-9-10-19(3,4)17(21)15(25)18(26-13(2)23)22(16,7)27-20/h8,15-18,25H,1,9-12H2,2-7H3/t15-,16+,17-,18-,20-,21+,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inactive analog of forskolin; does not activate adenylyl cyclase. |

1,9-Dideoxyforskolin Dilution Calculator

1,9-Dideoxyforskolin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.642 mL | 13.21 mL | 26.4201 mL | 52.8402 mL | 66.0502 mL |
| 5 mM | 0.5284 mL | 2.642 mL | 5.284 mL | 10.568 mL | 13.21 mL |
| 10 mM | 0.2642 mL | 1.321 mL | 2.642 mL | 5.284 mL | 6.605 mL |
| 50 mM | 0.0528 mL | 0.2642 mL | 0.5284 mL | 1.0568 mL | 1.321 mL |
| 100 mM | 0.0264 mL | 0.1321 mL | 0.2642 mL | 0.5284 mL | 0.6605 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demethylluvangetin
Catalog No.:BCN7569
CAS No.:64652-10-4
- VcMMAE
Catalog No.:BCC2033
CAS No.:646502-53-6
- H-D-Glu(OMe)-OH
Catalog No.:BCC2940
CAS No.:6461-04-7
- Sinigrin monohydrate
Catalog No.:BCN2595
CAS No.:64550-88-5
- Acantrifoside E
Catalog No.:BCN6646
CAS No.:645414-25-1
- Oxprenolol hydrochloride
Catalog No.:BCC7630
CAS No.:6452-73-9
- Scoulerine
Catalog No.:BCN6623
CAS No.:6451-73-6
- Isovanillic acid
Catalog No.:BCN3376
CAS No.:645-08-9
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Rubianthraquinone
Catalog No.:BCN6880
CAS No.:644967-44-2
- Cefotaxime sodium
Catalog No.:BCC8908
CAS No.:64485-93-4
- Glycoursodeoxycholic acid
Catalog No.:BCN7369
CAS No.:64480-66-6
- Coleonol B
Catalog No.:BCN4188
CAS No.:64657-21-2
- Demethylwedelolactone
Catalog No.:BCN2663
CAS No.:6468-55-9
- Dihydroflavokawain C
Catalog No.:BCC9229
CAS No.:
- Cinaciguat hydrochloride
Catalog No.:BCC8096
CAS No.:646995-35-9
- Morin dihydrate
Catalog No.:BCN8156
CAS No.:6472-38-4
- RA-V
Catalog No.:BCN3513
CAS No.:64725-24-2
- Tetrahydroalstonine
Catalog No.:BCN4189
CAS No.:6474-90-4
- 1,2,3,6,7-Pentamethoxyxanthone
Catalog No.:BCN7525
CAS No.:64756-86-1
- 6-Hydroxy-1,2,3,7-tetramethoxyxanthone
Catalog No.:BCN7565
CAS No.:64756-87-2
- L-trans-2,4-PDC
Catalog No.:BCC6595
CAS No.:64769-66-0
- Betulalbuside A
Catalog No.:BCN4190
CAS No.:64776-96-1
- Trilobatin 2''-acetate
Catalog No.:BCN4191
CAS No.:647853-82-5
Synthetic transformation of ptychantin into forskolin and 1,9-dideoxyforskolin.[Pubmed:16749796]
J Org Chem. 2006 Jun 9;71(12):4619-24.
Forskolin (1), a highly oxygenated labdane diterpenoid and an activator of adenylate cyclase, has been synthesized in 12 steps and 12% overall yield from ptychantin A (4), which has been isolated from liverwort Ptychanthus striatus in good yield. The 1alpha-hydroxy group was furnished by stereoselective reduction of the corresponding carbonyl group by sodium in t-BuOH. The 9alpha-hydroxy group was introduced stereoselectively by epoxidation of delta(9.11)-enolether. 1,9-Dideoxyforskolin (2), an inhibitor of glucose transporter, has been synthesized in 8 steps and 37% overall yield. The hydroxy group at C-1 was removed by solid-state thicarbonylimidazolation and subsequent radical cleavage.
Adenylate cyclase and potassium channels are involved in forskolin- and 1,9-dideoxyforskolin-induced inhibition of pregnant rat uterus contractility.[Pubmed:10739518]
Am J Obstet Gynecol. 2000 Mar;182(3):620-4.
OBJECTIVE: We sought to study the contribution of potassium channels in the effect of forskolin and 1,9-Dideoxyforskolin on uterine contractility in the pregnant rat. STUDY DESIGN: Rings taken from the middle portions of uterine horns from rats at 16 days of gestation were positioned in organ chambers containing physiologic salt solution bubbled with 5% carbon dioxide in air (37 degrees C, pH approximately 7.4) for isometric tension recording under 2 g passive tension. The effects of cumulative concentrations of forskolin and 1,9-Dideoxyforskolin in the absence or presence of an adenylate cyclase inhibitor (MDL-12,330A, 10(-5) mol/L), a nonselective potassium channel blocker (tetrabutylammonium, 10(-4) mol/L), or an adenosine triphosphate-dependent potassium channel blocker (glibenclamide 10(-5) mol/L) were studied. RESULTS: Both forskolin and, to a lesser extent, 1,9-Dideoxyforskolin inhibit uterine contractions. Tetrabutylammonium, glibenclamide, and MDL-12, 330A attenuated the effects of forskolin, whereas glibenclamide was less effective against 1,9-Dideoxyforskolin. CONCLUSION: Activation of adenylate cyclases, as well as adenosine triphosphate-dependent potassium channels and, to a greater extent, calcium-dependent potassium channels, is involved in the inhibitory effect of forskolin in uterine rings from rats at 16 days of gestation. Inhibition of uterine contractions by 1,9-Dideoxyforskolin is less than that by forskolin and involves activation of adenylate cyclase and calcium-dependent potassium channels. Whether activation of guanylate cyclase is involved in the effect of the agents on calcium-dependent potassium channels needs further investigation. 1, 9-Dideoxyforskolin is not an inactive isomer of forskolin in rat uterine rings.
Selective modulation of vinblastine sensitivity by 1,9-dideoxyforskolin and related diterpenes in multidrug resistant tumour cells.[Pubmed:8094975]
Br J Cancer. 1993 Mar;67(3):471-9.
The ability of 1,9-Dideoxyforskolin (DDF), 1-deoxyforskolin (DF) and forskolin to modulate cellular sensitivity to vinblastine (VBL) was examined in drug-sensitive parental KB-3-1 cells and a multidrug-resistant subline, KB-GRC1, derived by transfection of mdr1. Fifty microM DF and forskolin enhanced the 1 h uptake of VBL by 8.0 +/- 0.7 (s.d.) and 4.7 +/- 2.5-fold, respectively, with 50 microM DDF producing a 13.6 +/- 1.9-fold increase. The greater effect of DDF relative to forskolin indicated that the effect was independent of activation of cAMP, and this was supported by a lack of effect of dibutyryl cAMP on the uptake. The effect of these agents on uptake were < or = 1.4-fold in KB-3-1 cells. DDF selectively inhibited initial efflux in cells expressing a functional P-glycoprotein (PGP), but both forskolin and DDF inhibited the terminal phase of efflux irrespective of PGP expression. Neither agent affected membrane permeability of polarisation and forskolin did not enhance the uptake of VBL in protein-free liposomes. At a non-toxic concentration of 20 microM, DDF and forskolin decreased the IC50 of VBL from 18.9 to 2.7 and 13 nM in KB-GRC1 cells, respectively, and DDF acted synergistically with VBL as shown by median effect analysis [combination index = 0.20 +/- 0.05 (s.d.)]. In contrast, these diterpenes did not affect VBL sensitivity in KB-3-1 cells. These results indicate that the diterpenes modulate VBL sensitivity predominantly by inhibiting PGP-mediated efflux activity.
Forskolin's structural analogue 1,9-dideoxyforskolin has Ca2+ channel blocker-like action in rat cerebellar granule cells.[Pubmed:8804917]
Eur J Pharmacol. 1996 May 6;303(1-2):101-8.
Forskolin, routinely used as a specific activator of the cAMP pathway, is also a blocker of various ionic channels in a cAMP-independent way. We investigated, in rat cerebellar granule cells in culture, the effects of forskolin and its structural analogue 1,9-Dideoxyforskolin on Ca2+ entry. Changes in cytosolic free Ca2+ concentration ([Ca]i) were monitored using fura-2 microfluorimetry. The increase in [Ca]i observed in response to membrane depolarization by 30 mM KCI was reduced by 20% in the presence of 100 microM forskolin, and by 71% with the same concentration of 1,9-Dideoxyforskolin. A dose-response curve for 1,9-Dideoxyforskolin gave an estimated IC50 of 54 microM. Additional experiments using the patch-clamp technique showed that 100 microM 1,9-Dideoxyforskolin inhibit voltage-activated Ca2+ currents by 63%, although forskolin had no significant effect in the same conditions. This blocking effect of 1,9-Dideoxyforskolin is not specific of a given Ca2+ channel type.
Partial reversal of doxorubicin resistance by forskolin and 1,9-dideoxyforskolin in murine sarcoma S180 variants.[Pubmed:2825978]
Cancer Res. 1988 Feb 1;48(3):539-43.
Acquired resistance to chemotherapeutic agents is an important clinical problem. One preclinical model, termed multidrug resistance (MDR), is characterized by a complex phenotype of cross-resistance to biochemically unrelated antineoplastic agents, the presence of a high-molecular-weight membrane glycoprotein, and impaired accumulation of drug. To determine whether MDR is mediated in part by altered cyclic 3',5'-adenosine monophosphate (cAMP) levels, the effect of incubation with the adenylate cyclase agonist, forskolin, was investigated in the murine sarcoma S180 cell line and two MDR variants (A5-.8, A5-2.5). Basal cAMP levels in sensitive and MDR lines were not significantly different (range, 0.15 +/- 0.05 to 0.31 +/- 0.09 pmol/mg protein); however, 1-h incubation with forskolin, 10 microM, elevated intracellular cAMP 2-fold in the parent line and 43- and 35-fold in the variants. The adenylate cyclase agonists, prostaglandin E2 and cholera toxin, and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine had no significant effect on cAMP levels. To determine the effect of forskolin on doxorubicin-induced cell lethality, S180 and MDR lines were incubated with doxorubicin plus forskolin for 1 h and cloned in soft agar. Coincubation with forskolin partially reversed doxorubicin resistance in the MDR lines in a dose-dependent fashion. To determine whether this effect was mediated solely by elevation of intracellular cAMP, the inactive 1,9-dideoxy analogue of forskolin (DF) was used. Incubation with DF resulted in no elevation of cAMP levels in the sensitive or resistant cell lines; however, DF also partially reversed doxorubicin resistance in the MDR variants. Furthermore, coincubation of the A5-2.5 cell line with doxorubicin and 8-bromo cAMP, 1 mM, did not result in reversal of resistance to doxorubicin. To determine whether the reversal of resistance by the diterpenes was associated with alteration of doxorubicin transport, uptake and efflux of [14C]doxorubicin were measured. Coincubation with both forskolin and DF, 10 microM, enhanced [14C]doxorubicin uptake in the resistant cells, while drug efflux was significantly affected only in the cell line exhibiting intermediate resistance. Since both forskolin and its inactive analogue are effective in partially reversing resistance to doxorubicin and augmenting anthracycline uptake, a mechanism other than elevation of cAMP is most likely responsible.
Direct anesthetic-like effects of forskolin on the nicotinic acetylcholine receptors of PC12 cells.[Pubmed:3005280]
J Biol Chem. 1986 Mar 5;261(7):3103-6.
Forskolin is thought to be a highly specific activator of adenyl cyclase. However, when applied to rat pheochromocytoma (PC12) cells at concentrations of 1 microM or higher it caused an immediate, concentration-dependent inhibition of carbachol-stimulated uptake of 86Rb+ through the nicotinic receptors, which did not appear to be related to activation of adenyl cyclase. The inhibition of receptor activation occurred instantaneously whereas cellular cAMP content did not increase for a measureable period of time. Normal receptor function was recovered rapidly upon removal of forskolin. Additional evidence that this effect of forskolin was not related to cAMP was obtained when 1,9-Dideoxyforskolin (an analog of forskolin which does not activate adenyl cyclase) also caused a rapid, concentration-dependent, rapidly reversible inhibition of receptor-mediated influx of 86Rb+ into the cells. An examination of the effect of forskolin on 86Rb+ uptake at various concentrations of carbachol showed that forskolin was not acting by competing with carbachol for the receptor activation site. Given the lipophilic nature of forskolin, it probably acts like a general anesthetic to perturb the plasma membrane lipid structure and alter the function of the nicotinic acetylcholine receptors, possibly by increasing the rate of closure of open channels.


