Corydalis yanhusuo
Corydalis yanhusuo
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Corydalis yanhusuo
- Cat.No. Product Name CAS Number COA
-
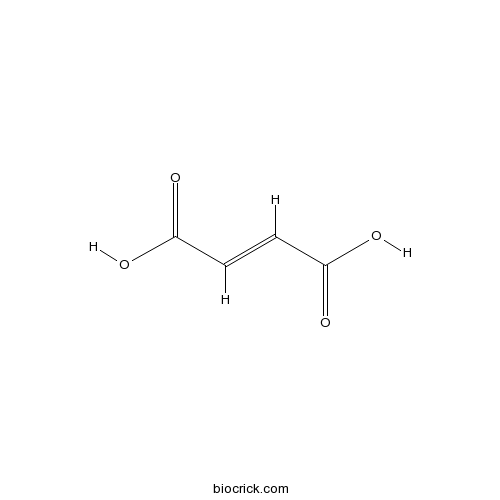
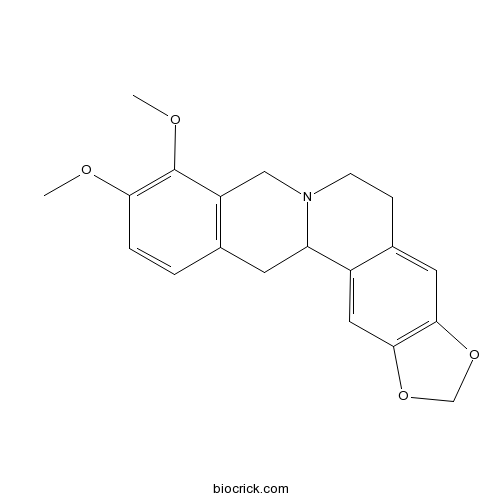
BCN5989
Fumaric acid110-17-8
Instructions
-
BCC8952
Docosanoic acid112-85-6
Instructions

-
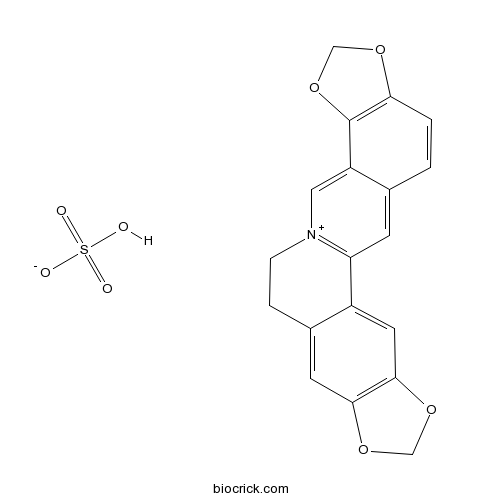
BCN2286
Coptisine sulfate1198398-71-8
Instructions
-
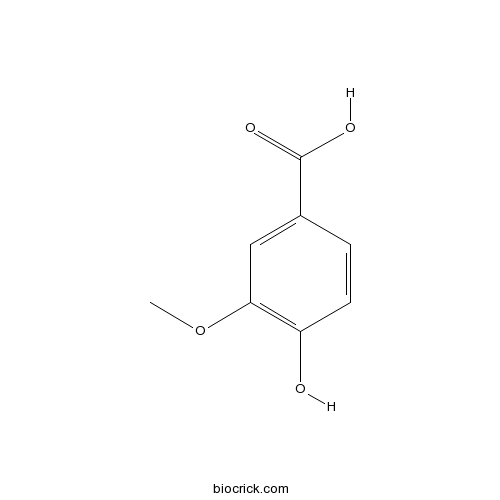
BCN6105
Vanillic acid121-34-6
Instructions
-
BCN6165
Protopine130-86-9
Instructions
-
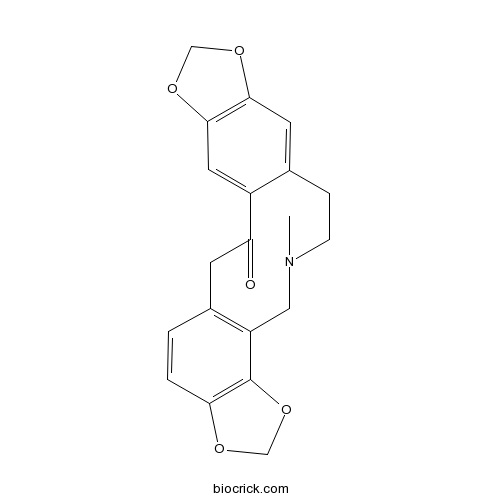
BCN6310
Tetrahydropalmatine2934-97-6
Instructions
-
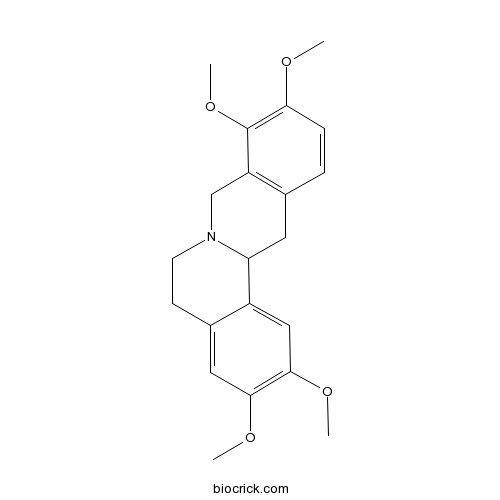
BCN2474
Dehydrocorydalin30045-16-0
Instructions
-
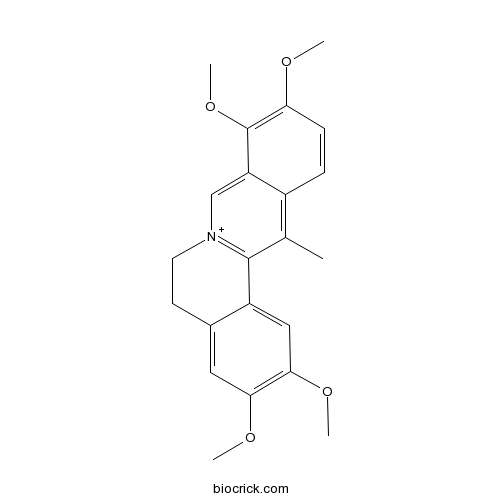
BCN5285
Palmatine3486-67-7
Instructions

-
BCN2334
D-Tetrahydropalmatine3520-14-7
Instructions

-
BCC5325
9-Hydroxycalabaxanthone hydrate483-14-7
Instructions

-
BCN2723
(-)-Isocorypalmine483-34-1
Instructions

-
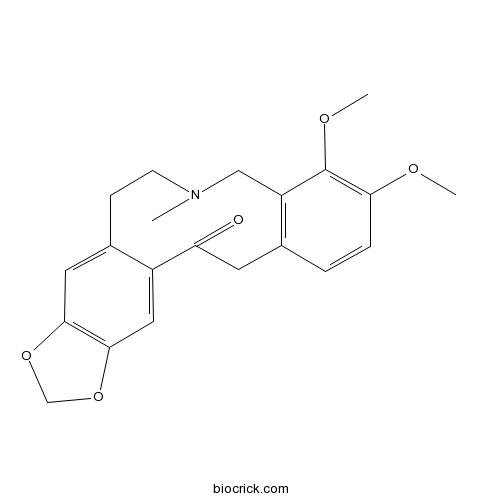
BCN9043
Allocryptopine485-91-6
Instructions
-
BCN2342
Corydaline518-69-4
Instructions

-
BCN2648
Tetrahydroberberine522-97-4
Instructions
-
BCN2273
Dihydrochelerythrine6880-91-7
Instructions

-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Online screening of acetylcholinesterase inhibitors in natural products using monolith-based immobilized capillary enzyme reactors combined with liquid chromatography-mass spectrometry.[Pubmed: 29866504]
In order to develop a direct and reliable method for discovering lead compounds from traditional Chinese medicines (TCMs), a comparative online ligand fishing platform was developed using immobilized capillary enzyme reactors (ICERs) in combination with liquid chromatography-mass spectrometry (LC-MS). Methacrylate-based monolithic capillaries (400 μm I.D. × 10 cm) containing epoxy reactive groups were used as support to immobilize the target enzyme acetylcholinesterase (AChE). The activity and kinetic parameters of the AChE-ICER were investigated using micro-LC-UV. Subsequently, ligand fishing and identification from mixtures was carried out using the complete AChE-ICER-LC-MS platform. For efficient distinction of true actives from false positives, highly automated comparative analyses were run alternatingly using AChE-ICERs and negative control-ICERs, both online installed in the system. After washing unbound compounds to the waste, bound ligands were eluted from the AChE-ICER to a trapping loop using a denaturing solution. The trapped ligands were further separated and identified using LC-MS. Non-specific binding to the monolith support or non-functional sites of the immobilized enzyme was investigated by exposing analytes to the negative control-ICER. The specificity of the proposed approach was verified by analyzing a known AChE inhibitor in the presence of an inactive compound. The platform was applied to screen for AChE inhibitors in extracts of Corydalis yanhusuo. Eight compounds (columbamine, jatrorrhizine, coptisine, palmatine, berberine, dehydrocorydaline, tetrahydropalmatine and corydaline) with AChE binding affinity were detected and identified, and their AChE inhibitory activities were further verified by an in vitro enzymatic inhibition assay. Experimental results show that the proposed comparative online ligand fishing platform is suitable for rapid screening and mass-selective detection of AChE inhibitors in complex mixtures.
Development and Validation of a HPLC-ESI-MS/MS Method for Simultaneous Quantification of Fourteen Alkaloids in Mouse Plasma after Oral Administration of the Extract of Corydalis yanhusuo Tuber: Application to Pharmacokinetic Study.[Pubmed: 29561801]
None
Metabolic profiles of corydaline in rats by ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry.[Pubmed: 29235899]
1. Corydaline, an isoquinoline alkaloid obtained from the rhizomes of Corydalis yanhusuo, exhibits anti-acetylcholinesterase, anti-angiogenic, anti-allergic and gastric-emptying activities. In this study, a rapid and reliable ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF-MS) method was developed and employed for the comprehensive study of the metabolites of corydaline in rats. 2. Altogether, 43 metabolites were identified in the plasma (11), bile (9), urine (34) and feces (21) of rats after oral administration of corydaline at a dose of 4.5mg/kg. 3. It was demonstrated that demethylation, hydroxylation, sulfation and glucuronidation were the major metabolic transformation pathways. Among these, two metabolites were identified as tetrahydropalmatine and isocorybulbine, and 33 phase I and phase II products were inferred to be new metabolites arising from the in vivo metabolism of corydaline. 4. Importantly, this research provides scientific and reliable support for full understanding of the metabolic profiles of corydaline and the results could help to elucidate its safety and efficacy.


