Ganoderma lucidum
Ganoderma lucidum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
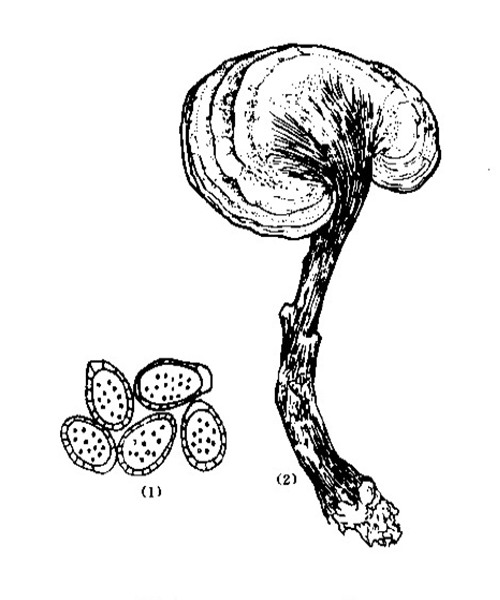
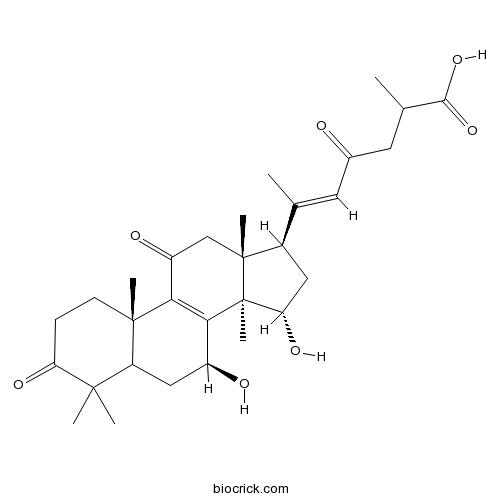
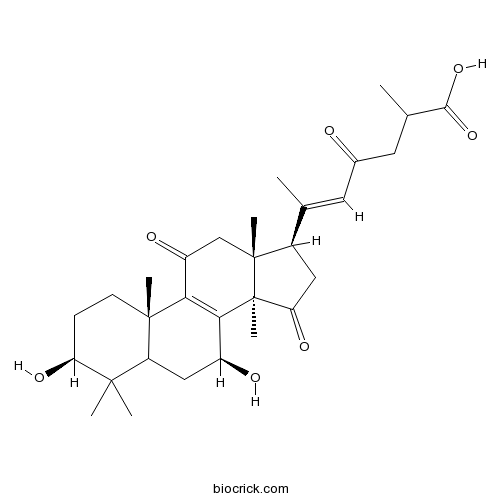
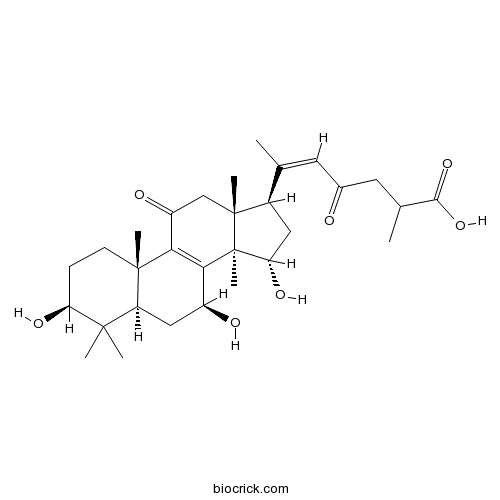
Natural products/compounds from Ganoderma lucidum
- Cat.No. Product Name CAS Number COA
-
BCN3208
Ganoderenic acid A100665-40-5
Instructions
-
BCN7966
Ganoderenic acid B100665-41-6
Instructions
-
BCN3210
Ganoderenic acid C100665-42-7
Instructions
-
BCN2445
Ganoderenic acid D100665-43-8
Instructions
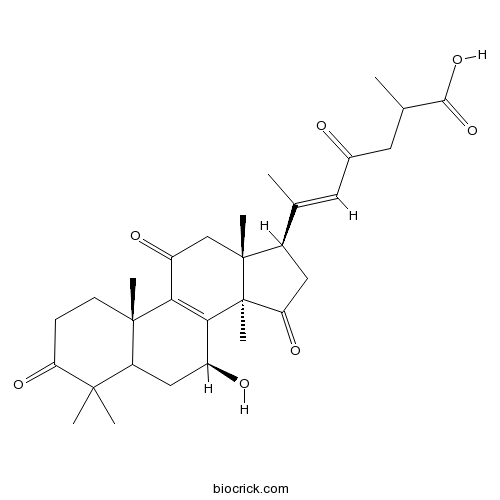
-
BCN2870
Deacetyl ganoderic acid F100665-44-9
Instructions
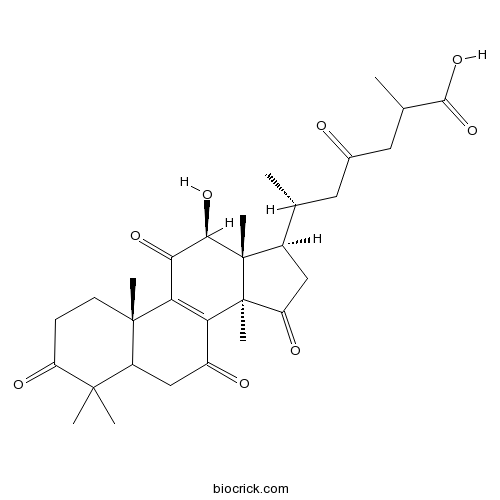
-
BCN2872
Ganolactone B1028449-53-7
Instructions
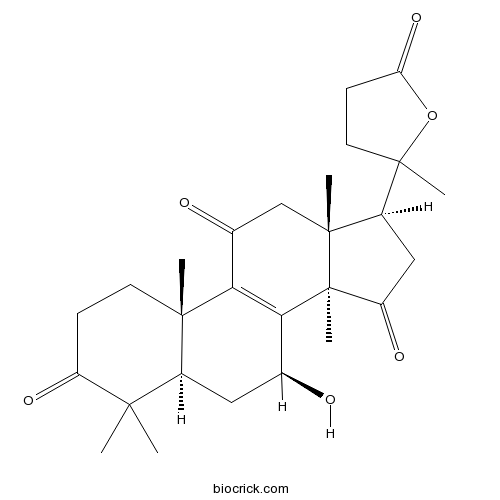
-
BCN3036
Ganoderic acid C2103773-62-2
Instructions
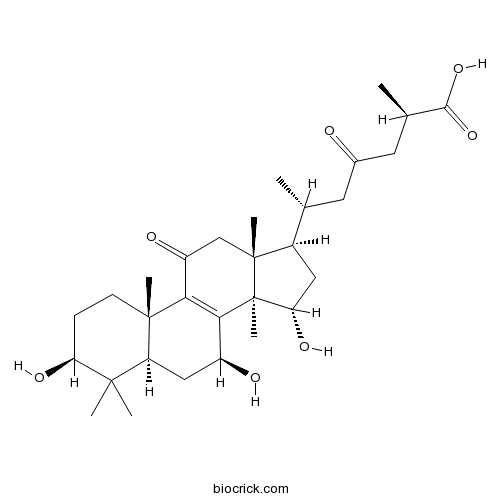
-
BCN5859
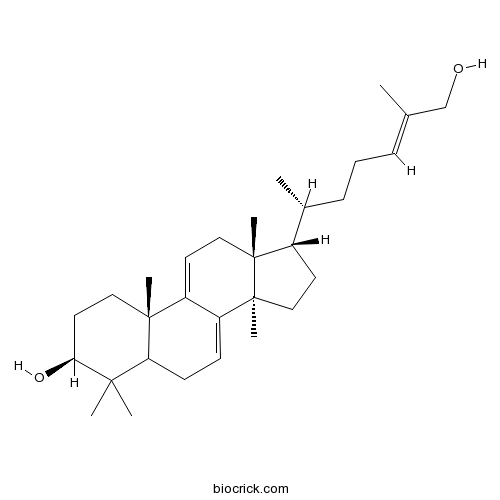
Ganoderol B104700-96-1
Instructions
-
BCN2451
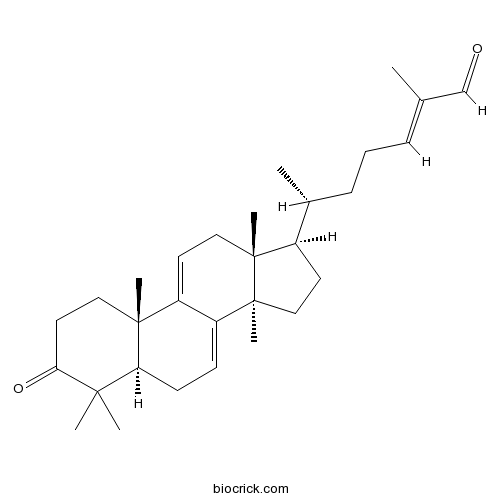
Ganoderal A104700-98-3
Instructions
-
BCN3257
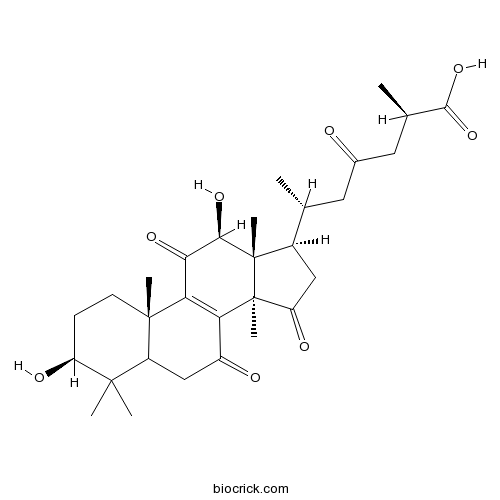
Ganoderic acid C6105742-76-5
Instructions
-
BCN5879
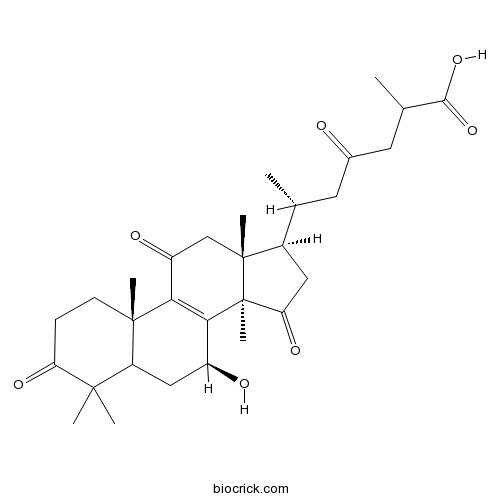
Ganodermanondiol107900-76-5
Instructions

-
BCN2437
Ganoderic acid D108340-60-9
Instructions
-
BCN2438
Ganoderic acid N110241-19-5
Instructions

-
BCN8241
Ganoderenic acid E110241-23-1
Instructions
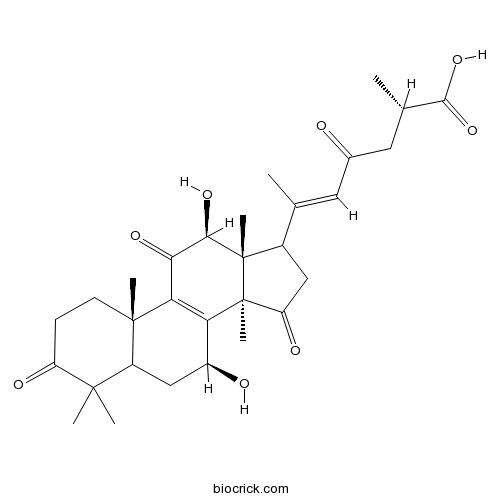
-
BCC8643
3β,7β,15β-trihydroxy-11-oxo-lanosta-8-en-24->20 lactone1694587-15-9
Instructions
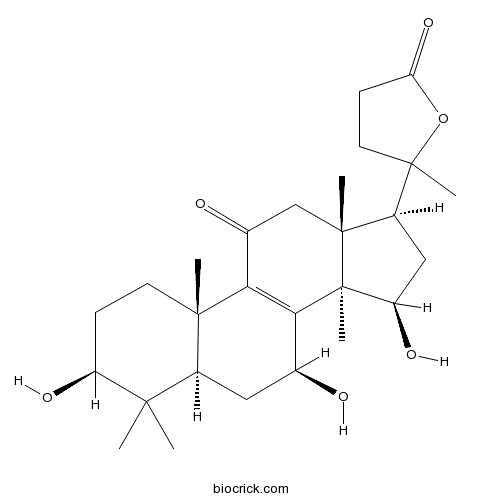
-
BCN7849
Ganoderlactone D1801934-15-5
Instructions
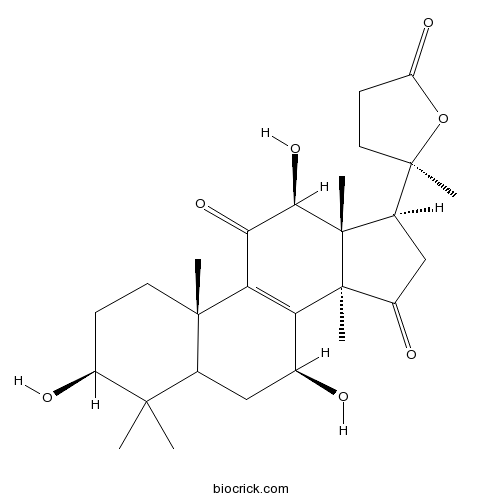
-
BCN9080
Lanosta-8,20(22)-dien-26-oic acid, 15-hydroxy-3,11,23-trioxo-, (15α,20Z)-1961358-01-9
Instructions

-
BCN9083
(3β,7β,12β,20Z )- 3,7,12- trihydroxy-11,15,23-trioxo-lanost-8,20-dien-26-oic acid1961358-02-0
Instructions
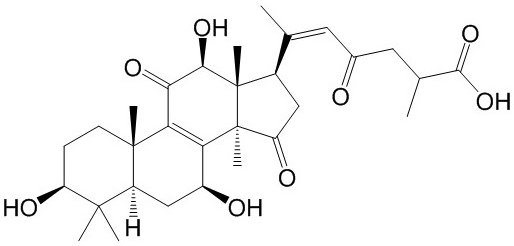
-
BCN8231
20-Hydroxyganoderic acid G400604-12-8
Instructions

-
BCN2442
Ganoderic acid LM2508182-41-0
Instructions

-
BCN8390
Myristic acid544-63-8
Instructions

-
BCN3034
Ganoderic acid B81907-61-1
Instructions

-
BCN3033
Ganoderic acid A81907-62-2
Instructions

-
BCN2940
20(21)-Dehydrolucidenic acid A852936-69-7
Instructions

-
BCN9081
Chol-8-en-24-oic acid, 7,15-dihydroxy-4,4,14-trimethyl-3,11-dioxo-, (5α)-942936-54-1
Instructions

-
BCN8051
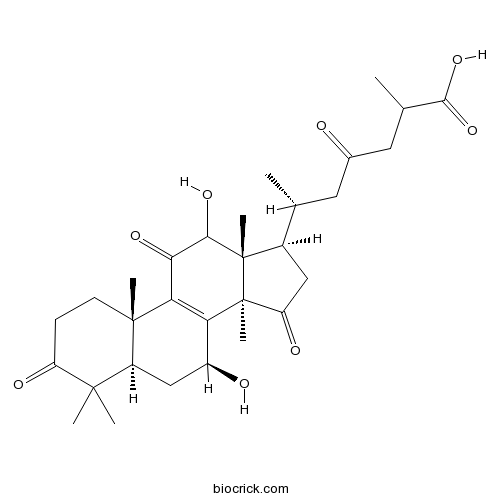
12-Hydroxyganoderic acid D942950-96-1
Instructions
-
BCN2715
Lucidenic acid A95311-94-7
Instructions

-
BCN3035
Ganoderic acid C195311-97-0
Instructions

-
BCN8242
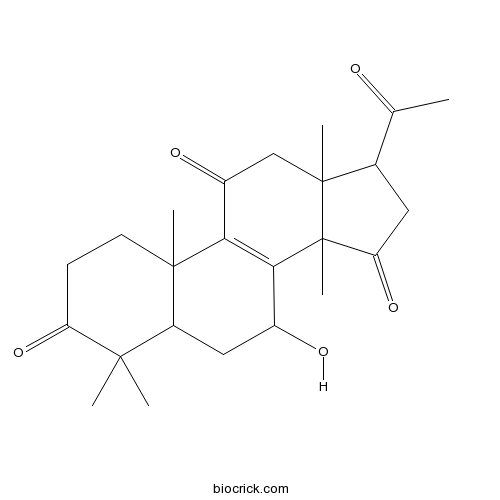
Lucidone B97653-93-5
Instructions
-
BCC8989
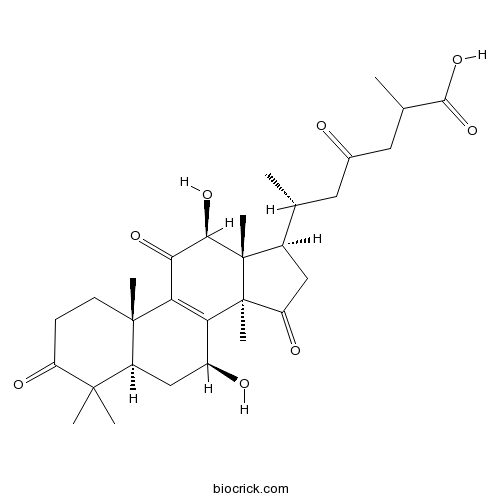
Ganoderic acid D297653-94-6
Instructions
-
BCN3038
Ganoderic acid H98665-19-1
Instructions

-
BCN2865
Ganoderic acid I98665-20-4
Instructions

-
BCN2915
Ganoderic acid G98665-22-6
Instructions

Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement.[Pubmed: 30114634]
Ganoderma lucidum comprises probably 400 different biologically active constituents principally polysaccharides, triterpenoids, proteins, enzymes, steroids, sterols, nucleotides, fatty acids, vitamins and minerals which have been proved to have several therapeutical properties to control various diseases. Broad spectrum of its pharmacological actions have been established which include immunomodulation, anticancer, antidiabetic, antioxidant, antiatherosclerotic, antifibrotic, chemopreventive, antitumor, anticancer drug toxicity prevention, analgesic, anti inflammatory, antinociceptive, antimicrobial, hypolipidemic, hepatoprotective, antiandrogenic, antiangiogenic, antiherpetic, antiarthritic, antiosteoporotic, antiaging, antiulcer properties and estrogenic activity. The present review is an effort to investigate and compile the reported biologically active compounds, pharmacological activity, toxicity and comparative study of different medicinal mushrooms.
Metabolism of ganoderic acids by a Ganoderma lucidum cytochrome P450 and the 3-keto sterol reductase ERG27 from yeast.[Pubmed: 30077898]
Ganoderic acids, a group of oxygenated lanostane-type triterpenoids, are the major bioactive compounds produced by the well-known medicinal macro fungus Ganoderma lucidum. More than 150 ganoderic acids have been identified, and the genome of G. lucidum has been sequenced recently. However, the biosynthetic pathways of ganoderic acids have not yet been elucidated. Here, we report the functional characterization of a cytochrome P450 gene CYP512U6 from G. lucidum, which is involved in the ganoderic acid biosynthesis. CYP512U6 hydroxylates the ganoderic acids DM and TR at the C-23 position to produce hainanic acid A and ganoderic acid Jc, respectively. In addition, CYP512U6 can also hydroxylate a modified ganoderic acid DM in which the C-3 ketone has been reduced to hydroxyl by the sterol reductase ERG27 from Saccharomyces cerevisiae. An NADPH-dependent cytochrome P450 reductase from G. lucidum was also isolated and characterized. These results will help elucidate the biosynthetic pathways of ganoderic acids.
Medicinal Mushrooms Supplements Alter Chicken Intestinal Microbiome.[Pubmed: 30055560]
Chicken intestinal microbiomes are known to enhance chicken health and increase production. Identifying natural supplements that shift bacteria communities in favor of beneficial bacteria is critical. This study is a preliminary investigation of the use of medicinal mushrooms nutritional supplements (MSs) including Lentinus edodes, Ganoderma lucidum, Pleurotus ostreatus, and Cordyceps sp. to modulate bacteria in chicken guts. Two-hundred seventy 1-day-old broilers were weighed and randomly assigned to nine different treatments containing MSs. Treatments were assigned according to MS incubation times (14-56 days) and levels of inclusion (5% and 10%) into chicken feed as follows: (1) control MS0; (2) MS1, 14 days, 5%; (3) MS2, 14 days, 10%; (4) MS3, 28 days, 5%; (5) MS4, 28 days, 10%; (6) MS5, 42 days, 5%; (7) MS6, 42 days, 10%; (8) MS7, 56 days, 5%; and (9) MS8, 56 days, 10%. Changes in bacterial community in the stomach (S) and bursa (B) of experimental chickens were assessed using a next-generation sequencing approach. Results indicated that all MSs, except MS1-S (14 days, 5%) and MS4-S (28 days, 10%), completely eliminated Mollicutes, a class of stomach pathogens. MS7 (56 days, 5%) reduced Clostridia 4.8-fold and 3-fold in the stomach and bursa, respectively. The chicken stomach contained far more diverse bacteria than the bursa. Whereas the overall diversity of bacteria decreased with MSs, there was no consistent pattern with incubation time and inclusion level. The abundance of Gammaproteobacteria and unidentified species increased tremendously in both organs, while Bacilli were generally reduced. MS7 (56 days, 5%) showed the most promising result but needs further research. This study high-lights potential benefits from the use of fungal-based supplements as health enhancers in chicken production.
Ultra-Low Freezing to Preserve the Lingzhi or Reishi Medicinal Mushroom Ganoderma lucidum (Agaricomycetes).[Pubmed: 30055559]
Ganoderma lucidum (Curtis) P. Karst., commonly used in traditional Chinese medicine, is characterized by strong genetic and phenotypic variability that reflects its active components. To preserve such a source of pharmacologically active metabolites, specimens must be collected from different geographic regions and their genetic integrity ensured during storage. To this aim, we tested the effect of ultra-low freezing (ULF) at -120°C on the vitality, mycelial growth rate, and fruiting ability of 3 Italian strains of G. lucidum. Results showed that all strains reacted positively to ULF, demonstrating an ability to recover after 3 months of storage without morphological or physiological changes occurring, regardless of treatment. The successful storage of G. lucidum at -120°C opens up the possibility to create a germplasm bank to collect strains of this medicinal fungus from throughout Europe, thereby contributing to the maintenance of its diversity.
Phenolics, tocopherols and fatty acid profiling of wild and commercial mushrooms from Pakistan.[Pubmed: 30043568]
Mushrooms can be used as nutraceutical or functional foods to maintain and promote good health. In the present study, wild Ganoderma lucidum and four commercial mushrooms, Pleurotus ostreatus, Volvariella volvacea, Hericium erinaceus and Lentinus edodes, collected from Pakistan were screened for phenolics, tocopherols and fatty acid contents. High performance liquid chromatography analysis of phenolic acids showed that chlorogenic acid, ferulic acid, gallic acid, p-Coumaric and caffeic acids were observed in selected mushrooms. H. erinaceus contained high amounts of chlorogenic acid (11.49±0.1 µ/g of dry weight) and ferulic acid (7.84±0.7 µg/g of dry weight). γ-tocopherol and lutein were present in all studied mushrooms. Lutein contents were higher in H. erinaceus (2.42±0.087 µg/g of DW) followed by V. volvacea> P. ostreatus> L. edodes. γ-tocopherol was observed in the range of 74.25±3.01 to 29.65±1.2 µg/g of dry weight. GC/MS analysis of fatty acids showed that linoleic acid (18:2n6c), oleic acid (18:1n9c), palmitic acid (C16:0), stearic acid (C18:0), linolenic acid (18:3n3) and nonadecanoic acid (C19-0), were the main fatty acids found in selected mushrooms. The unsaturated fatty acids were predominated over saturated fatty acids. It is concluded that selected mushrooms are good sources of antioxidant compounds and unsaturated fatty acids.


