Panax notoginseng
Panax notoginseng
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
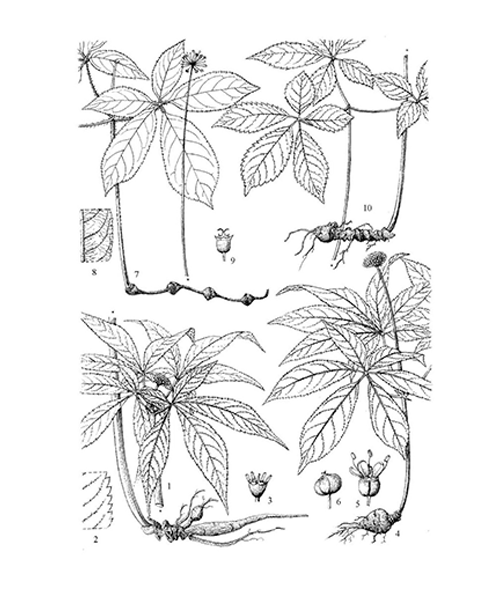
Natural products/compounds from Panax notoginseng
- Cat.No. Product Name CAS Number COA
-
BCN1072
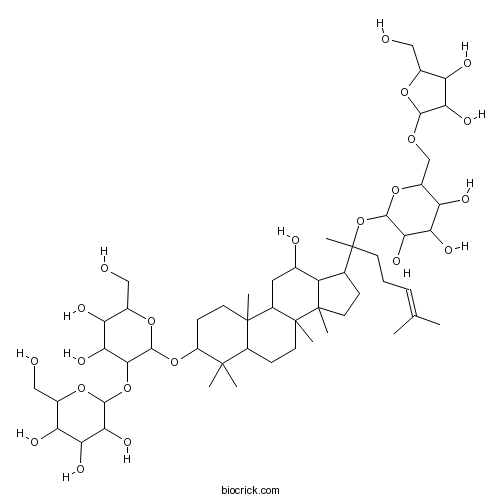
Ginsenoside Rc11021-14-0
Instructions
-
BCN2484
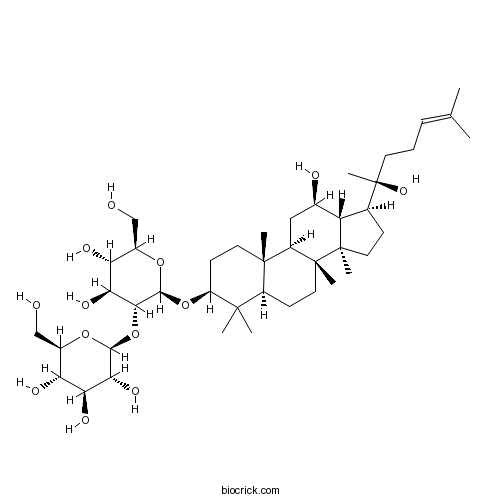
20(R)-Ginsenoside Rh2112246-15-8
Instructions

-
BCN1068
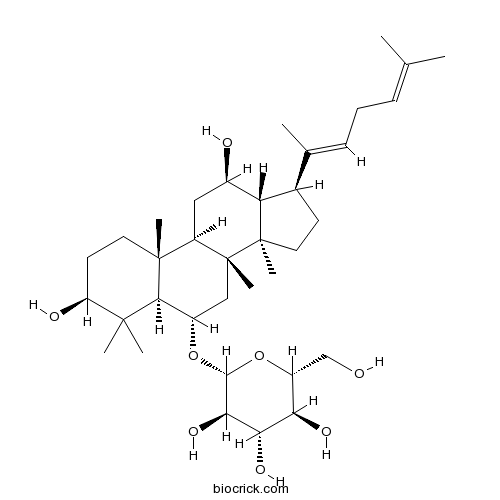
Ginsenoside Rg314197-60-5
Instructions
-
BCN3503
Ginsenoside Rh4174721-08-5
Instructions
-
BCN2771
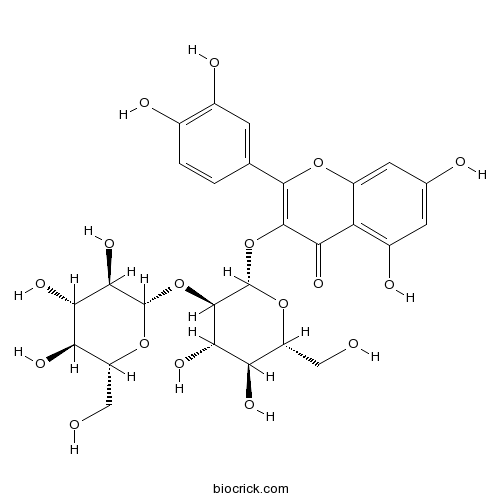
Quercetin-3-O-sophoroside18609-17-1
Instructions
-
BCN3551
Ginsenoside Rg5186763-78-0
Instructions

-
BCN1080
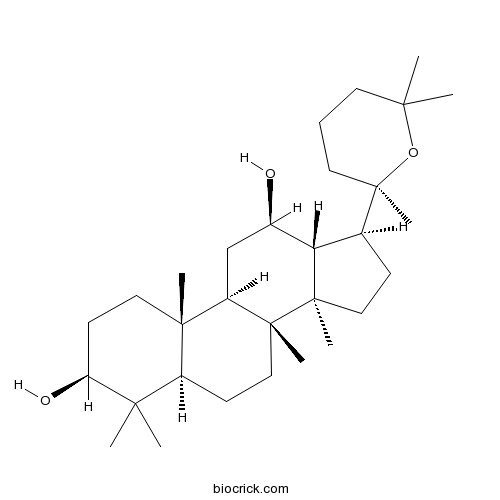
Panaxadiol19666-76-3
Instructions
-
BCN4865
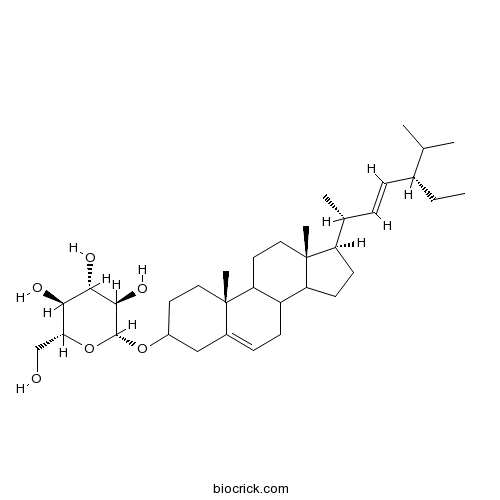
Stigmasterol glucoside19716-26-8
Instructions
-
BCN1066
Ginsenoside Rg122427-39-0
Instructions

-
BCN5065
Falcarindiol225110-25-8
Instructions

-
BCN1254
(20S)-Protopanaxdiol30636-90-9
Instructions

-
BCN1081
Panaxatriol32791-84-7
Instructions

-
BCN3502
Ginsenoside Rk3364779-15-7
Instructions

-
BCN5018
20(R)-Ginsenoside Rg338243-03-7
Instructions

-
BCN1063
Ginsenoside Rb141753-43-9
Instructions

-
BCN3552
Ginsenoside Rk1494753-69-4
Instructions

-
BCN1073
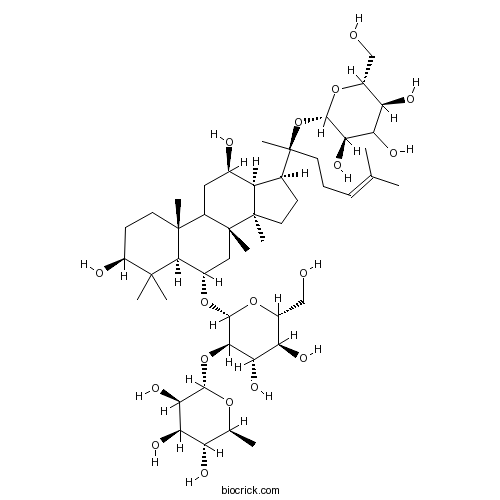
Ginsenoside Re52286-59-6
Instructions
-
BCN1067
Ginsenoside Rg252286-74-5
Instructions

-
BCN1074
Ginsenoside Rd52705-93-8
Instructions

-
BCX0482
Dencichine5302-45-4
Instructions

-
BCN1244
Ginsenoside F153963-43-2
Instructions

-
BCN5944
Liquiritin551-15-5
Instructions

-
BCN5946
Liquiritigenin578-86-9
Instructions

-
BCN5796
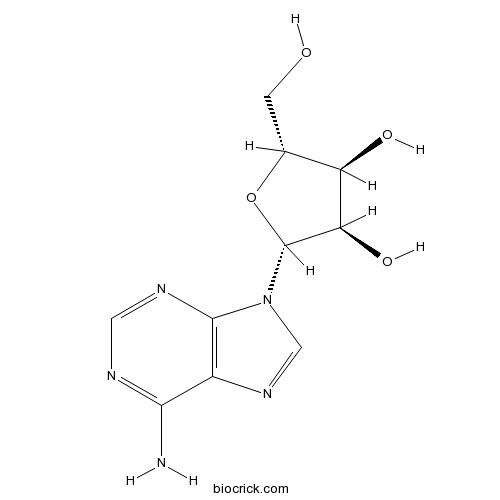
Adenosine58-61-7
Instructions
-
BCN1245
Ginsenoside F262025-49-4
Instructions

-
BCN1069
Ginsenoside Rh163223-86-9
Instructions
-
BCN1065
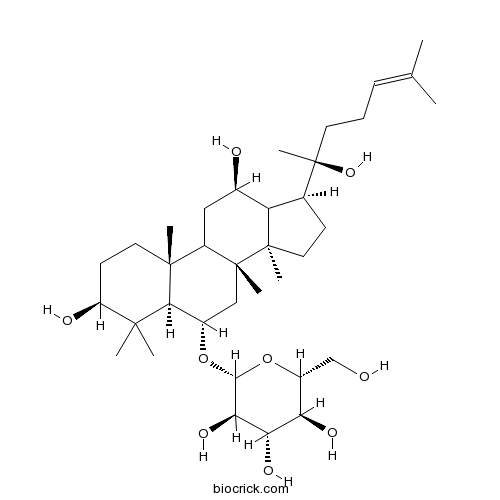
Ginsenoside Rb368406-26-8
Instructions

-
BCN1062
Pseudoginsenoside F1169884-00-0
Instructions
-
BCN1078
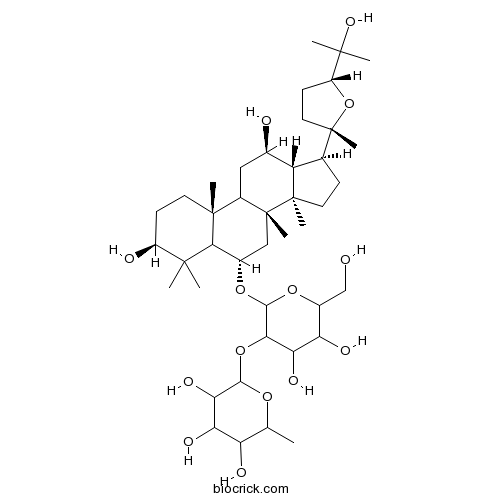
(20R)-Protopanaxdiol7755-01-3
Instructions

-
BCN1070
20(S)-Ginsenoside Rh278214-33-2
Instructions

-
BCN2339
Gypenoside XVII80321-69-3
Instructions

-
BCN1097
Notoginsenoside R180418-24-2
Instructions

-
BCN3328
Notoginsenoside R280418-25-3
Instructions

-
BCN3700
(20R)-Ginsenoside Rh180952-71-2
Instructions

-
BCN3854
Notoginsenoside Fa88100-04-3
Instructions

-
BCN3852
Notoginsenoside Fe88105-29-7
Instructions

Drechmeria panacis sp. nov., an endophyte isolated from Panax notoginseng.[Pubmed: 30113296]
None
Phytochemistry, pharmacology, and clinical use of Panax notoginseng flowers buds.[Pubmed: 30088301]
Panax notoginseng is a well-known traditional Chinese medicine, and dried flower buds of P. notoginseng (FBP) have also been used as a medicine or tea for a long time. The pharmacological effects of FBP include antihypertensive, anticancer, hepatoprotective, and cardiovascular protective effects. The compounds in FBP include saponins, flavonoids, volatile oils, and polysaccharides. The total saponins are the principal bioactive components. In modern applications, FBP is used to treat hypertension and tinnitus. There have been many studies on FBP and its effects in recent years, and it has attracted much attention in the medical field. This review summarizes the chemical components, pharmacological action, and clinical uses of FBP comprehensively to provide the references of deeper studies.
Rapid identification and quantification of Panax notoginseng with its adulterants by near infrared spectroscopy combined with chemometrics.[Pubmed: 30077893]
Traditional methods for identification of Panax notoginseng (PN) such as high performance liquid chromatography (HPLC) and gas chromatography (GC) are time-consuming, laborious and difficult to realize rapid and online analysis. In this research, the feasibility of identification and quantification of PN with rhizoma curcumae (RC), Curcuma longa (CL) and rhizoma alpiniae offcinarum (RAO) are investigated by using near infrared (NIR) spectroscopy combined with chemometrics. Five chemical pattern recognition methods including hierarchical cluster analysis (HCA), partial least squares-discriminant analysis (PLS-DA), artificial neural networks (ANN), support vector machine (SVM) and extreme learning machine (ELM) are used to build identification model of the dataset with 109 samples of PN and its three adulterants. Then seven datasets of binary, ternary and quaternary adulterations of PN are designed, respectively. Five multivariate calibration methods, i.e., principal component regression (PCR), support vector regression (SVR), partial least squares regression (PLSR), ANN and ELM are used to build quantitative model and compared for each dataset, separately. Finally, in order to further improve the prediction accuracy, SG smoothing, 1st derivative, 2nd derivative, continuous wavelet transform (CWT), standard normal variate (SNV), multiple scatter correction (MSC) and their combinations are investigated. Results show that PLS-DA and SVM can achieve 100% classification accuracy for identification of 109 PN with its three adulterants. PLSR is an optimal calibration method by comprehensive consideration of prediction accuracy, over-fitting and efficiency for the quantitative analysis of seven adulterated datasets. Furthermore, the predictive ability of the PLSR model for PN contents can be improved obvious by pretreating the spectra by the optimal preprocessing method, with correlation coefficients of which all higher than 0.99.
Nototronesides A-C, Three Triterpene Saponins with a 6/6/9 Fused Tricyclic Tetranordammarane Carbon Skeleton from the Leaves of Panax notoginseng.[Pubmed: 30020793]
Three triterpene saponins, nototronesides A-C (1-3), possessing an unprecedented 6/6/9 fused tricyclic tetranordammarane core, were isolated from the leaves of Panax notoginseng. Their structures were elucidated on the basis of spectroscopic data, and the structure of sapogenin (1a) was further confirmed by X-ray crystallography. The existence of 1-3 adds a new dimension to the diversity of the triterpene family. Moreover, compound 2 showed a moderate neuroprotective effect on serum deficiency-induced cellular damage in PC12 cells.
Impacts of silicon addition on arsenic fractionation in soils and arsenic speciation in Panax notoginseng planted in soils contaminated with high levels of arsenic.[Pubmed: 30015185]
None
Affiliation with Natural Products at KIB of Prof. Zhou Jun: On the Occasion of 80th Anniversary of Kunming Institute of Botany, CAS.[Pubmed: 30014450]
Prof. Zhou Jun, Academician of Chinese Academy of Sciences (1999), is a phytochemist and medicinal chemist of China. He is one of the pioneers of Kunming Institute of Botany, CAS and a major founder of the State Key Laboratory of Phytochemistry and Plant Resources in West China. The chemical compositions of some TCM from genus of Dioscorea, Aconitum, Panax, Paris, Cynanchum, Gastrodia, Dendrobium etc. and family Asclepiadaceae, Caryophyllaceae, Hypoxidaceae etc. have been explored by Prof. Zhou's team as steroids, triterpenoids, alkaloids, cyclic peptides and phenols etc., which revealed the main active composition of those TCM such as Panax notoginseng, Paris yunnanensis and Gastrodia elata.
Notoginsenoside R1 promotes the growth of neonatal rat cortical neurons via the Wnt/β-catenin signaling pathway.[Pubmed: 29992896]
Notoginsenoside R1 (NGR1) is one of the main effective components of Panax notoginseng. Primary cortical neurons were harvested from neonatal rats and cultured to analyze the role of NGR1 in neuronal growth and the effects of NGR1 on the Wnt/β-catenin signaling pathway. Following treatment with NGR1, immunocytochemistry was used to detect expression of Tuj1 and MAP2, and RT-qPCR was used to measure mRNA levels of key factors in the Wnt signaling pathway. Results showed that NGR1 promotes growth of cultured neurons and significantly up-regulates mRNA levels of β-catenin, Dishevelled, and Frizzled. To further confirm whether NGR1 promoted cortical neuron growth via the Wnt/β-catenin signaling pathway, we knocked down β-catenin mRNA by siRNA interference; following NGR1 treatment of β-catenin-knockdown neurons, β-catenin mRNA levels increased significantly. In conclusion, these results demonstrate that NGR1 promotes growth of cultured cortical neurons from the neonatal rat, possibly via the Wnt/β-catenin signaling pathway.


