p-Hydroxyphenethyl trans-ferulateCAS# 84873-15-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84873-15-4 | SDF | Download SDF |
| PubChem ID | 637308 | Appearance | Powder |
| Formula | C18H18O5 | M.Wt | 314.3 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
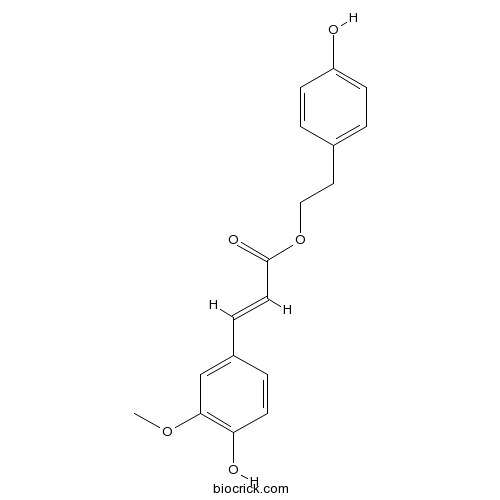
| Chemical Name | 2-(4-hydroxyphenyl)ethyl (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)OCCC2=CC=C(C=C2)O)O | ||
| Standard InChIKey | JMSFLLZUCIXALN-WEVVVXLNSA-N | ||
| Standard InChI | InChI=1S/C18H18O5/c1-22-17-12-14(4-8-16(17)20)5-9-18(21)23-11-10-13-2-6-15(19)7-3-13/h2-9,12,19-20H,10-11H2,1H3/b9-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. P-Hydroxyphenethyl trans-ferulate exhibits affinity toward 5-HT(7) receptors in a competitive binding assay. 2. P-Hydroxyphenethyl trans-ferulate can double quinone reductase specific activity in Hepa 1c1c7 cells at a level of 2.1 microg/mL (6.6 microM). |
| Targets | 5-HT Receptor |

p-Hydroxyphenethyl trans-ferulate Dilution Calculator

p-Hydroxyphenethyl trans-ferulate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1817 mL | 15.9084 mL | 31.8167 mL | 63.6335 mL | 79.5418 mL |
| 5 mM | 0.6363 mL | 3.1817 mL | 6.3633 mL | 12.7267 mL | 15.9084 mL |
| 10 mM | 0.3182 mL | 1.5908 mL | 3.1817 mL | 6.3633 mL | 7.9542 mL |
| 50 mM | 0.0636 mL | 0.3182 mL | 0.6363 mL | 1.2727 mL | 1.5908 mL |
| 100 mM | 0.0318 mL | 0.1591 mL | 0.3182 mL | 0.6363 mL | 0.7954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gnetin D
Catalog No.:BCN3380
CAS No.:84870-53-1
- BIIB021
Catalog No.:BCC2124
CAS No.:848695-25-0
- Stigmasta-4,22,25-trien-3-one
Catalog No.:BCN4396
CAS No.:848669-09-0
- Stigmasta-4,25-dien-3-one
Catalog No.:BCN4395
CAS No.:848669-08-9
- P11
Catalog No.:BCC6275
CAS No.:848644-86-0
- A 582941
Catalog No.:BCC7920
CAS No.:848591-90-2
- Vasicinolone
Catalog No.:BCN4394
CAS No.:84847-50-7
- NCH 51
Catalog No.:BCC2422
CAS No.:848354-66-5
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- SSR128129E
Catalog No.:BCC4498
CAS No.:848318-25-2
- EX-527 R-enantiomer
Catalog No.:BCC5595
CAS No.:848193-69-1
- EX-527 S-enantiomer
Catalog No.:BCC5594
CAS No.:848193-68-0
- Pulsatilla camphor
Catalog No.:BCN8184
CAS No.:90921-11-2
- Betulinic acid 3β-O-alpha-L-rhamnopyranosyl-(1->2)-[β-D-glucopyranosyl-(1->4)]-alpha-L-arabinopyranoside
Catalog No.:BCC8303
CAS No.:848784-87-2
- AZD8931 (Sapitinib)
Catalog No.:BCC3734
CAS No.:848942-61-0
- UFP-101
Catalog No.:BCC5775
CAS No.:849024-68-6
- Floribundasaponin A
Catalog No.:BCN1330
CAS No.:37341-36-9
- Glaucogenin C mono-D-thevetoside
Catalog No.:BCN7089
CAS No.:849201-84-9
- Foretinib (GSK1363089)
Catalog No.:BCC1263
CAS No.:849217-64-7
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
- Chinensine B
Catalog No.:BCN4397
CAS No.:849245-34-7
- Grape Seed Extract
Catalog No.:BCC5317
CAS No.:84929-27-1
- 1-(4-Fluorobenzyl)-2-chlorobenzimidazole
Catalog No.:BCC8407
CAS No.:84946-20-3
- TMPH hydrochloride
Catalog No.:BCC7360
CAS No.:849461-91-2
Isolation and identification of phase II enzyme-inducing agents from nonpolar extracts of green onion (Allium spp.).[Pubmed:17061815]
J Agric Food Chem. 2006 Nov 1;54(22):8417-24.
The objective of the study was to isolate and identify potential cancer preventive constituents from green onion based on the ability to induce quinone reductase (QR, a representative phase II enzyme) in murine hepatoma cells (Hepa 1c1c7). Crude nonpolar solvent extracts were prepared from freeze-dried green onion by sequential refluxing with hexane and then ethyl acetate, followed by liquid-liquid extraction. Active fractions were subjected to the Hepa 1c1c7 bioassay-guided steps of flash chromatography, thin layer chromatography (TLC), and high-pressure preparative liquid chromatography (HPLC) to afford pure isolates capable of inducing QR. Multiple fractions were active in inducing QR. Five pure compounds were isolated from active fractions and identified using spectroscopic methods; these were p-Hydroxyphenethyl trans-ferulate (1), 5,6-dimethyl-2-pyridinecarboxylic acid (2), ferulic acid (3), 1-(6-hydroxy-[3]pyridyl)-propan-1-one (4), and N-trans-feruloyl 3-O-methyldopamine (5). p-Hydroxyphenethyl trans-ferulate (1) doubled QR specific activity in Hepa 1c1c7 cells at a level of 2.1 microg/mL (6.6 microM).
Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis.[Pubmed:16643021]
J Nat Prod. 2006 Apr;69(4):536-41.
Serotonin receptor (5-HT(7)) binding assay-directed fractionation of a methanol extract of the dried roots of Angelica sinensis led to the isolation and identification of 21 compounds including a new phenolic ester, angeliferulate (1), and three new phthalides, 10-angeloylbutylphthalide (2), sinaspirolide (3), and ansaspirolide (4), along with 17 known compounds, p-Hydroxyphenethyl trans-ferulate (5), Z-ligustilide (6), Z-butylidenephthalide (7), senkyunolide I (8), Z-6-hydroxy-7-methoxydihydroligustilide (9), N-butylbenzenesulfonamide (10), 11(S),16(R)-dihydroxyoctadeca-9Z,17-diene-12,14-diyn-1-yl acetate (11), (3R,8S)-falcarindiol (12), heptadeca-1-en-9,10-epoxy-4,6-diyne-3,8-diol (13), oplopandiol (14), 8-hydroxy-1-methoxy-, Z-9-heptadecene-4,6-diyn-3-one (15), imperatorin, ferulic acid, vanillin, stigmasterol, sucrose, and 1,3-dilinolenin. This is the first report of a sulfonamide (10) identified from a higher plant source, although its presence needs further investigation. Biosynthetic pathways for dimeric phthalides 3 and 4 are proposed. Compounds 5, 7, 11, 12, 15, and imperatorin exhibited affinity toward 5-HT(7) receptors in a competitive binding assay.


