10Z-HymenialdisinePan kinase inhibitor; potently inhibits Cdk1, Cdk2, Cdk3 and Cdk5 CAS# 82005-12-7 |
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82005-12-7 | SDF | Download SDF |
| PubChem ID | 3036831 | Appearance | Powder |
| Formula | C11H10BrN5O2 | M.Wt | 324.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SK&F 108752 | ||
| Solubility | Soluble to 10 mM in DMSO | ||
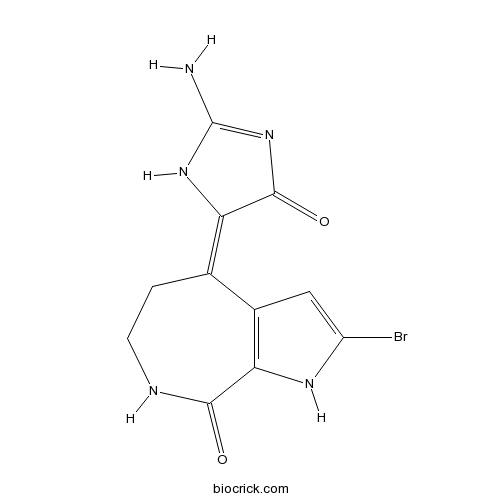
| Chemical Name | (4E)-4-(2-amino-4-oxo-1H-imidazol-5-ylidene)-2-bromo-1,5,6,7-tetrahydropyrrolo[2,3-c]azepin-8-one | ||
| SMILES | C1CNC(=O)C2=C(C1=C3C(=O)N=C(N3)N)C=C(N2)Br | ||
| Standard InChIKey | ATBAETXFFCOZOY-QPJJXVBHSA-N | ||
| Standard InChI | InChI=1S/C11H10BrN5O2/c12-6-3-5-4(7-10(19)17-11(13)16-7)1-2-14-9(18)8(5)15-6/h3,15H,1-2H2,(H,14,18)(H3,13,16,17,19)/b7-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pan kinase inhibitor (IC50 values are 6, 10, 22, 28, 35, 40, 70, 80, 100, 470, 500, 600, 700 and 700 nM for MEK1, GSK-3β, Cdk1/cyclin B, Cdk5/p25, CK1, Cdk2/cyclin A, Cdk2/cyclin E, ASK-γ, Cdk3/cyclin E, Erk1, PKCγ, Cdk4/cyclin D1, Cdk6/cyclin D2 and PKCα respectively.) Inhibits NF-κB activation and blocks IL-8 production in U937 cells (IC50 values are 1-2 and 0.34-0.48 μM respectively). |

10Z-Hymenialdisine Dilution Calculator

10Z-Hymenialdisine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0852 mL | 15.4259 mL | 30.8518 mL | 61.7036 mL | 77.1295 mL |
| 5 mM | 0.617 mL | 3.0852 mL | 6.1704 mL | 12.3407 mL | 15.4259 mL |
| 10 mM | 0.3085 mL | 1.5426 mL | 3.0852 mL | 6.1704 mL | 7.713 mL |
| 50 mM | 0.0617 mL | 0.3085 mL | 0.617 mL | 1.2341 mL | 1.5426 mL |
| 100 mM | 0.0309 mL | 0.1543 mL | 0.3085 mL | 0.617 mL | 0.7713 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Daurichromenic acid
Catalog No.:BCN4355
CAS No.:82003-90-5
- 8-Anilino-1-naphthalenesulfonic acid
Catalog No.:BCC8785
CAS No.:82-76-8
- Peri acid
Catalog No.:BCC9116
CAS No.:82-75-7
- Visnagin
Catalog No.:BCN4367
CAS No.:82-57-5
- 1-Amino-2-methylanthraquinone
Catalog No.:BCC8451
CAS No.:82-28-0
- Alpha-Toxicarol
Catalog No.:BCN6467
CAS No.:82-09-7
- Rottlerin
Catalog No.:BCC7127
CAS No.:82-08-6
- Benzanthrone
Catalog No.:BCC8845
CAS No.:82-05-3
- Khellin
Catalog No.:BCN4356
CAS No.:82-02-0
- Lancerin
Catalog No.:BCN2803
CAS No.:81991-99-3
- Vitedoin A
Catalog No.:BCN6741
CAS No.:819861-40-0
- Hyperxanthone E
Catalog No.:BCN8072
CAS No.:819860-76-9
- Boc-Leucinol
Catalog No.:BCC2724
CAS No.:82010-31-9
- Loteprednol etabonate
Catalog No.:BCC4916
CAS No.:82034-46-6
- NSC 33994
Catalog No.:BCC2441
CAS No.:82058-16-0
- JNJ 17203212
Catalog No.:BCC7668
CAS No.:821768-06-3
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
- FMK
Catalog No.:BCC1580
CAS No.:821794-92-7
- Andropanoside
Catalog No.:BCN4570
CAS No.:82209-72-1
- Andrographiside
Catalog No.:BCN4569
CAS No.:82209-76-5
- Atomoxetine HCl
Catalog No.:BCC5046
CAS No.:82248-59-7
- Impurity C of Alfacalcidol
Catalog No.:BCC5385
CAS No.:82266-85-1
- Breyniaionoside A
Catalog No.:BCN7112
CAS No.:823182-23-6
- Styraxlignolide F
Catalog No.:BCN3416
CAS No.:823214-06-8
Aldisine alkaloids from the Philippine sponge Stylissa massa are potent inhibitors of mitogen-activated protein kinase kinase-1 (MEK-1).[Pubmed:11784156]
J Med Chem. 2002 Jan 17;45(2):529-32.
Raf/MEK-1/MAPK cascade inhibitor activity-directed fractionation of the sponge Stylissa massa afforded eight known alkaloids: aldisine (1), 2-bromoaldisine (2), 10Z-debromohymenialdisine (3), 10E-hymenialdisine (4), 10Z-Hymenialdisine (5), hymenin (6), oroidin (7), and 4,5-dibromopyrrole-2-carbonamide (8). Both 4 and 5 showed significant enzyme inhibitory activity (IC(50) 3 and 6 nM, respectively). Secondary assays identified these compounds as potent MEK-1 inhibitors. Compounds 4 and 5 also inhibited the growth of human tumor LoVo cells.
Inhibition of cyclin-dependent kinases, GSK-3beta and CK1 by hymenialdisine, a marine sponge constituent.[Pubmed:10662688]
Chem Biol. 2000 Jan;7(1):51-63.
BACKGROUND: Over 2000 protein kinases regulate cellular functions. Screening for inhibitors of some of these kinases has already yielded some potent and selective compounds with promising potential for the treatment of human diseases. RESULTS: The marine sponge constituent hymenialdisine is a potent inhibitor of cyclin-dependent kinases, glycogen synthase kinase-3beta and casein kinase 1. Hymenialdisine competes with ATP for binding to these kinases. A CDK2-hymenialdisine complex crystal structure shows that three hydrogen bonds link hymenialdisine to the Glu81 and Leu83 residues of CDK2, as observed with other inhibitors. Hymenialdisine inhibits CDK5/p35 in vivo as demonstrated by the lack of phosphorylation/down-regulation of Pak1 kinase in E18 rat cortical neurons, and also inhibits GSK-3 in vivo as shown by the inhibition of MAP-1B phosphorylation. Hymenialdisine also blocks the in vivo phosphorylation of the microtubule-binding protein tau at sites that are hyperphosphorylated by GSK-3 and CDK5/p35 in Alzheimer's disease (cross-reacting with Alzheimer's-specific AT100 antibodies). CONCLUSIONS: The natural product hymenialdisine is a new kinase inhibitor with promising potential applications for treating neurodegenerative disorders.
The natural product hymenialdisine inhibits interleukin-8 production in U937 cells by inhibition of nuclear factor-kappaB.[Pubmed:9223588]
J Pharmacol Exp Ther. 1997 Jul;282(1):459-66.
The nuclear factor-kappaB (NF-kappaB) family of transcription factors have been implicated in the inducible expression of genes involved in inflammatory and immune responses. As such, a specific inhibitor of NF-kappaB would be a useful therapeutic agent in a variety of inflammatory disorders. The marine natural product hymenialdisine was evaluated as an inhibitor of NF-kappaB in U937 cells. U937 cells were transfected with either a luciferase reporter plasmid containing the human immunodeficiency virus long terminal repeat or the interleukin-8 (IL-8) core promoter, both of which are activated by NF-kappaB. Hymenialdisine caused a concentration-dependent decrease in luciferase production from both reporters when the cells were stimulated with tumor necrosis factor-alpha, lipopolysaccharide or phorbol myristate acetate. An electrophoretic mobility shift assay confirmed its activity by inhibiting DNA binding of NF-kappaB. Hymenialdisine was shown to be a selective inhibitor of NF-kappaB in that it had no effect on the binding of other transcription factors to their DNA concensus motifs; these included activator protein-1, CCAAT/enhancer binding protein and Sp1. Functional studies showed hymenialdisine to be an inhibitor of IL-8 production and IL-8 mRNA formation in the U937 cell. Investigation into the mechanism of action of hymenialdisine showed that it was not due to inhibition of protein kinase C because the selective protein kinase C inhibitor RO 32-0432 was inactive against tumor necrosis factor-alpha-stimulated luciferase and IL-8 production. The compound also had no effect on IkappaB alpha or IkappaB beta phosphorylation and degradation. Thus, hymenialdisine is a potent inhibitor of NF-kappaB and IL-8 production in U937 cells.


