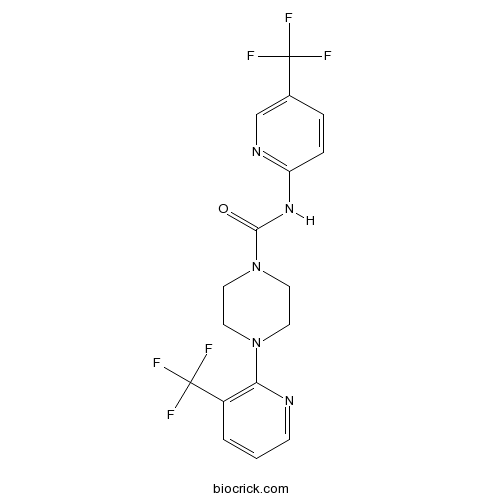
JNJ 17203212Reversible, competitive and potent TRPV1 antagonist CAS# 821768-06-3 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 821768-06-3 | SDF | Download SDF |
| PubChem ID | 11339118 | Appearance | Powder |
| Formula | C17H15F6N5O | M.Wt | 419.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (238.48 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[3-(trifluoromethyl)pyridin-2-yl]-N-[5-(trifluoromethyl)pyridin-2-yl]piperazine-1-carboxamide | ||
| SMILES | C1CN(CCN1C2=C(C=CC=N2)C(F)(F)F)C(=O)NC3=NC=C(C=C3)C(F)(F)F | ||
| Standard InChIKey | JFRYYGVYCWYIDQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H15F6N5O/c18-16(19,20)11-3-4-13(25-10-11)26-15(29)28-8-6-27(7-9-28)14-12(17(21,22)23)2-1-5-24-14/h1-5,10H,6-9H2,(H,25,26,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversible, competitive and potent TRPV1 antagonist (pKi values are 6.5, 7.1 and 7.3 at rat, guinea pig and human TRPV1 respectively). Inhibits capsaicin- and H+-induced channel activation (pIC50 values are 6.32 and 7.23 respectively) and exhibits antitussive and analgesic activity in vivo. |

JNJ 17203212 Dilution Calculator

JNJ 17203212 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3848 mL | 11.9241 mL | 23.8481 mL | 47.6963 mL | 59.6203 mL |
| 5 mM | 0.477 mL | 2.3848 mL | 4.7696 mL | 9.5393 mL | 11.9241 mL |
| 10 mM | 0.2385 mL | 1.1924 mL | 2.3848 mL | 4.7696 mL | 5.962 mL |
| 50 mM | 0.0477 mL | 0.2385 mL | 0.477 mL | 0.9539 mL | 1.1924 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2385 mL | 0.477 mL | 0.5962 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NSC 33994
Catalog No.:BCC2441
CAS No.:82058-16-0
- Loteprednol etabonate
Catalog No.:BCC4916
CAS No.:82034-46-6
- Boc-Leucinol
Catalog No.:BCC2724
CAS No.:82010-31-9
- 10Z-Hymenialdisine
Catalog No.:BCC5773
CAS No.:82005-12-7
- Daurichromenic acid
Catalog No.:BCN4355
CAS No.:82003-90-5
- 8-Anilino-1-naphthalenesulfonic acid
Catalog No.:BCC8785
CAS No.:82-76-8
- Peri acid
Catalog No.:BCC9116
CAS No.:82-75-7
- Visnagin
Catalog No.:BCN4367
CAS No.:82-57-5
- 1-Amino-2-methylanthraquinone
Catalog No.:BCC8451
CAS No.:82-28-0
- Alpha-Toxicarol
Catalog No.:BCN6467
CAS No.:82-09-7
- Rottlerin
Catalog No.:BCC7127
CAS No.:82-08-6
- Benzanthrone
Catalog No.:BCC8845
CAS No.:82-05-3
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
- FMK
Catalog No.:BCC1580
CAS No.:821794-92-7
- Andropanoside
Catalog No.:BCN4570
CAS No.:82209-72-1
- Andrographiside
Catalog No.:BCN4569
CAS No.:82209-76-5
- Atomoxetine HCl
Catalog No.:BCC5046
CAS No.:82248-59-7
- Impurity C of Alfacalcidol
Catalog No.:BCC5385
CAS No.:82266-85-1
- Breyniaionoside A
Catalog No.:BCN7112
CAS No.:823182-23-6
- Styraxlignolide F
Catalog No.:BCN3416
CAS No.:823214-06-8
- SN 2
Catalog No.:BCC6325
CAS No.:823218-99-1
- Coelonin
Catalog No.:BCN3600
CAS No.:82344-82-9
- Heliocoromandaline
Catalog No.:BCN2046
CAS No.:82354-33-4
- Heliocurassavicine
Catalog No.:BCN2049
CAS No.:82354-34-5
A novel TRPV1 receptor antagonist JNJ-17203212 attenuates colonic hypersensitivity in rats.[Pubmed:21132125]
Methods Find Exp Clin Pharmacol. 2010 Oct;32(8):557-64.
This study examined the efficacy of a novel TRPV1 antagonist, JNJ-17203212, in two experimental rat models that exhibit a hypersensitive visceral motor response (VMR) to colorectal distension (CRD). In the first model, intraluminal administration of acetic acid (1% solution) into the distal colon produced an acute colonic hypersensitivity. In the second model, intraluminal administration of 2,4,6-trinitrobenzenesulfonic acid (TNBS) into the distal colon produced a chronic, post-inflammatory colonic hypersensitivity 30 days post-TNBS administration. Throughout this study, colonic sensitivity was assessed via quantification of VMR to CRD in rats following a single, oral administration of JNJ-17203212 (3, 10 or 30 mg/kg) or vehicle. Intraluminal administration of acetic acid and TNBS resulted in increased VMR to CRD when compared to controls. In both groups, VMR to CRD was significantly reduced by administration of JNJ-17203212 at 30 mg/kg. The results of this study show that the selective TRPV1 antagonist, JNJ-17203212, reduces sensitivity to luminal distension in both an acute, noninflammatory and a chronic, post-inflammatory rodent model of colonic hypersensitivity. These data indicate that TRPV1 is involved in the pathogenesis of visceral hypersensitivity and that JNJ-17203212 may be a potential therapeutic agent for functional bowel disorders characterized by abdominal hypersensitivity, such as irritable bowel syndrome.
Pharmacology and antitussive efficacy of 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethyl-pyridin-2-yl)-amide (JNJ17203212), a transient receptor potential vanilloid 1 antagonist in guinea pigs.[Pubmed:17690251]
J Pharmacol Exp Ther. 2007 Nov;323(2):665-74.
Transient receptor potential vanilloid 1 (TRPV1) plays an integral role in modulating the cough reflex, and it is an attractive antitussive drug target. The purpose of this study was to characterize a TRPV1 antagonist, 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethyl-pyridin-2-yl)-amide (JNJ17203212), against the guinea pig TRPV1 receptor in vitro followed by a proof-of-principle study in an acid-induced model of cough. The affinity of JNJ17203212 for the recombinant guinea pig TRPV1 receptor was estimated by radioligand binding, and it was functionally characterized by antagonism of low-pH and capsaicin-induced activation of the ion channel (fluorometric imaging plate reader and electrophysiology). The nature of antagonism was further tested against the native channel in isolated guinea pig tracheal rings. Following pharmacokinetic characterization of JNJ17203212 in guinea pigs, pharmacodynamic and efficacy studies were undertaken to establish the antitussive efficacy of the TRPV1 antagonist. The pK(i) of JNJ17203212 for recombinant guinea pig TRPV1 was 7.14 +/- 0.06. JNJ17203212 inhibited both pH (pIC(50) of 7.23 +/- 0.05) and capsaicin (pIC(50) of 6.32 +/- 0.06)-induced channel activation. In whole-cell patch clamp, the pIC(50) for inhibition of guinea pig TRPV1 was 7.3 +/- 0.01. JNJ17203212 demonstrated surmountable antagonism in isolated trachea, with a pK(B) value of 6.2 +/- 0.1. Intraperitoneal administration of 20 mg/kg JNJ17203212 achieved a maximal plasma exposure of 8.0 +/- 0.4 microM, and it attenuated capsaicin evoked coughs with similar efficacy to codeine (25 mg/kg). Last, JNJ17203212 dose-dependently produced antitussive efficacy in citric acid-induced experimental cough in guinea pigs. Our data provide preclinical support for developing TRPV1 antagonists for the treatment of cough.
Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain.[Pubmed:15788769]
J Neurosci. 2005 Mar 23;25(12):3126-31.
Cancer colonization of bone leads to the activation of osteoclasts, thereby producing local tissue acidosis and bone resorption. This process may contribute to the generation of both ongoing and movement-evoked pain, resulting from the activation of sensory neurons that detect noxious stimuli (nociceptors). The capsaicin receptor TRPV1 (transient receptor potential vanilloid subtype 1) is a cation channel expressed by nociceptors that detects multiple pain-producing stimuli, including noxious heat and extracellular protons, raising the possibility that it is an important mediator of bone cancer pain via its capacity to detect osteoclast- and tumor-mediated tissue acidosis. Here, we show that TRPV1 is present on sensory neuron fibers that innervate the mouse femur and that, in an in vivo model of bone cancer pain, acute or chronic administration of a TRPV1 antagonist or disruption of the TRPV1 gene results in a significant attenuation of both ongoing and movement-evoked nocifensive behaviors. Administration of the antagonist had similar efficacy in reducing early, moderate, and severe pain-related responses, suggesting that TRPV1 may be a novel target for pharmacological treatment of chronic pain states associated with bone cancer metastasis.
Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist.[Pubmed:15771431]
J Med Chem. 2005 Mar 24;48(6):1857-72.
High throughput screening using the recombinant human TRPV1 receptor was used to identify a series of pyridinylpiperazine ureas (3) as TRPV1 vanilloid receptor ligands. Exploration of the structure-activity relationships by parallel synthesis identified the essential pharmacophoric elements for antagonism that permitted further optimization via targeted synthesis to provide a potent orally bioavailable and selective TRPV1 modulator 41 active in several in vivo models.


