BenzanthroneCAS# 82-05-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82-05-3 | SDF | Download SDF |
| PubChem ID | 6697 | Appearance | Powder |
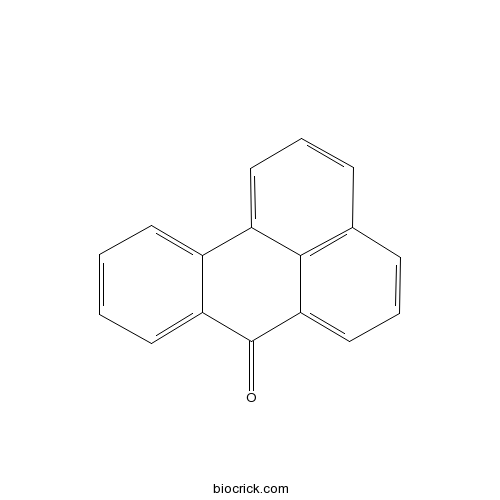
| Formula | C17H10O | M.Wt | 230.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | benzo[a]phenalen-7-one | ||
| SMILES | C1=CC=C2C(=C1)C3=CC=CC4=C3C(=CC=C4)C2=O | ||
| Standard InChIKey | HUKPVYBUJRAUAG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H10O/c18-17-14-8-2-1-7-12(14)13-9-3-5-11-6-4-10-15(17)16(11)13/h1-10H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzanthrone Dilution Calculator

Benzanthrone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3422 mL | 21.7108 mL | 43.4216 mL | 86.8432 mL | 108.5541 mL |
| 5 mM | 0.8684 mL | 4.3422 mL | 8.6843 mL | 17.3686 mL | 21.7108 mL |
| 10 mM | 0.4342 mL | 2.1711 mL | 4.3422 mL | 8.6843 mL | 10.8554 mL |
| 50 mM | 0.0868 mL | 0.4342 mL | 0.8684 mL | 1.7369 mL | 2.1711 mL |
| 100 mM | 0.0434 mL | 0.2171 mL | 0.4342 mL | 0.8684 mL | 1.0855 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Khellin
Catalog No.:BCN4356
CAS No.:82-02-0
- Lancerin
Catalog No.:BCN2803
CAS No.:81991-99-3
- Vitedoin A
Catalog No.:BCN6741
CAS No.:819861-40-0
- Hyperxanthone E
Catalog No.:BCN8072
CAS No.:819860-76-9
- KW-2478
Catalog No.:BCC2127
CAS No.:819812-04-9
- Clovin
Catalog No.:BCN7817
CAS No.:81970-00-5
- Splendoside
Catalog No.:BCN6647
CAS No.:81969-41-7
- 4(15),5,10(14)-Germacratrien-1-ol
Catalog No.:BCN4354
CAS No.:81968-62-9
- Z-Ligustilide
Catalog No.:BCC9193
CAS No.:81944-09-4
- Zofenopril calcium
Catalog No.:BCC5229
CAS No.:81938-43-4
- Polyphyllin H
Catalog No.:BCN2834
CAS No.:81917-50-2
- Momordicoside I aglycone
Catalog No.:BCN4353
CAS No.:81910-41-0
- Rottlerin
Catalog No.:BCC7127
CAS No.:82-08-6
- Alpha-Toxicarol
Catalog No.:BCN6467
CAS No.:82-09-7
- 1-Amino-2-methylanthraquinone
Catalog No.:BCC8451
CAS No.:82-28-0
- Visnagin
Catalog No.:BCN4367
CAS No.:82-57-5
- Peri acid
Catalog No.:BCC9116
CAS No.:82-75-7
- 8-Anilino-1-naphthalenesulfonic acid
Catalog No.:BCC8785
CAS No.:82-76-8
- Daurichromenic acid
Catalog No.:BCN4355
CAS No.:82003-90-5
- 10Z-Hymenialdisine
Catalog No.:BCC5773
CAS No.:82005-12-7
- Boc-Leucinol
Catalog No.:BCC2724
CAS No.:82010-31-9
- Loteprednol etabonate
Catalog No.:BCC4916
CAS No.:82034-46-6
- NSC 33994
Catalog No.:BCC2441
CAS No.:82058-16-0
- JNJ 17203212
Catalog No.:BCC7668
CAS No.:821768-06-3
Novel luminescent dyes for confocal laser scanning microscopy used in Trematoda parasite diagnostics.[Pubmed:30148507]
Acta Biochim Pol. 2018;65(3):449-454.
Benzanthrone derivates are now widely used in many industrial and scientific applications as dyes for polymers and textiles. In biochemical, biomedical and diagnostics investigations Benzanthrone dyes are used as a lipophilic fluorescent probe since many Benzanthrone derivates demonstrate bright fluorescence and they have ability to intercalate between membrane lipids. The aim of research presented here was to assess the luminescence ability of Benzanthrone derivatives using microscopic visualization of biological objects. Accordingly, specimens of freshwater trematodes: Diplostomum spathaceum, Diplodiscus subclavatus and Prosotocus confusus, were stained by novel Benzanthrone dyes using different fixatives. The samples were examined under a confocal laser scanning microscope. All of the dyes tested demonstrated good results for digestive and reproductive system visualization. Based on obtained results we conclude that Benzanthrone dyes could be used for internal and external structure confocal laser scanning microscopic imaging of trematode specimens.
Photophysical properties of benzanthrone derivatives: effect of substituent, solvent polarity and hydrogen bonding.[Pubmed:29561048]
Photochem Photobiol Sci. 2018 Apr 18;17(4):453-464.
Benzanthrone derivatives are potential fluorescent probes for various chemical and biological environments. A mechanistic understanding of their photophysical properties is pivotal for designing an efficient fluorescence sensor based on the Benzanthrone framework. In this study, we report on the effect of chemical substitution on the photophysical properties of two Benzanthrone derivatives, namely, 3-(N'-methyl)-piperazino-7H-benzo[de]anthracen-7-one [Me-PBA] and 3-(N'-phenyl)-piperazino-7H-benzo[de]anthracen-7-one [Ph-PBA] in different solvents and solvent mixtures of varying polarities and proticities. Both Benzanthrone derivatives show interesting solvent-dependent photophysical properties. Although both derivatives exhibit strong intramolecular charge transfer (ICT) characteristics in the excited state, the extent of the charge transfer is significantly influenced by the nature of the chemical substitution. Modulation of photophysical parameters as a function of solvent properties led us to propose that ICT is affected by solvent polarity and hydrogen bonding. From the viscosity effect, it is revealed that the weaker emission of Ph-PBA compared to Me-PBA in polar solvents is primarily due to the non-radiative torsional motion of the phenyl group in the former derivative. In protic solvents, intermolecular hydrogen bonding imparts strong non-radiative deactivation to both derivatives, thus rendering a weak fluorescence yield.
Species differences between rat and human in vitro metabolite profile, in vivo predicted clearance, CYP450 inhibition and CYP450 isoforms that metabolize benzanthrone: Implications in risk assessment.[Pubmed:29126801]
Food Chem Toxicol. 2018 Jan;111:94-101.
Benzanthrone (BNZ) is a polycyclic aromatic hydrocarbon found in industrial effluent causing skin, respiratory, gastrointestinal, genitourinary, nervous and hemopoietic toxicity. While its toxicity has been well studied, its metabolism in humans has not been investigated. The aim of this study was to characterize species differences in the in vitro metabolism of BNZ in rat and human liver microsomes and to identify the CYP isoforms involved in its metabolism. Upon incubation in liver microsomes, BNZ was found to be a direct substrate of phase I metabolism in both rat and human, undergoing oxidation and reduction. The Km in rat, 11.62 +/- 1.49 muM, was two-fold higher than humans (5.97 +/- 0.83 muM) suggesting higher affinity for human CYPs. Further, incubation with human rCYPs, BNZ was found to be substrate of multiple CYPs. The predicted in vivo hepatic clearance was 63.55 and 18.91 mL/min/kg in rat and human, respectively, indicating BNZ to be a high clearance compound. BNZ was found to be a moderate inhibitor of human CYP1A2. BNZ metabolism by multiple CYPs indicates that single enzyme genetic polymorphism is unlikely to have profound effect on the toxicokinetics of BNZ and default uncertainty factor of 3.16 might be sufficient to capture the intraspecies kinetic variability.
A novel function of TLR4 in mediating the immunomodulatory effect of Benzanthrone, an environmental pollutant.[Pubmed:28495615]
Toxicol Lett. 2017 Jul 5;276:69-84.
Our prior studies have reported that Benzanthrone (BA) manifests inflammatory responses in the spleen of Balb/c mice. The present investigation was carried out to study the impact of BA on macrophages, which are the primary scavenger cells in the body that act as a connecting link between innate and adaptive immunity. Parenteral administration of BA (daily for one week) to mice resulted in enhanced levels of nitric oxide (NO) and overexpression of inflammatory markers (COX-2, MMP-9 and PGE-2) in macrophages; however the level of MHC class-I and MHC class-II receptors were down regulated. Further, the potential membrane receptor targets (TLRs) of BA and its interaction with TLRs was investigated using computational methods. Professional phagocytes play pivotal roles in sensing bacteria through pathogen-associated molecular patterns (PAMPs) by various pathogen recognition receptors (PRRs), including Toll-like receptors (TLRs). Several studies have implicated these TLRs in the amplification of the inflammatory responses, however the fundamental role played by TLRs in mediating the inflammation associated with xenobiotics is still obscure and not understood. From the in silico analysis, it was evident that BA showed the highest binding affinity with TLR4 as compared to other TLRs. The western blotting studies confirmed that BA exposure indeed upregulated the expression of TLR 4, 5 and 9. Moreover, the downstream signaling cascade proteins of TLRs such as myeloid differentiation primary response protein-88 (MyD88), IL-1 receptor associated kinase (IRAK-1), and TNFR-associated factor (TRAF-6) were found to be enhanced in the BA treated groups. It was also observed that BA treatment increased the expression of ICAM-1, p-Lyn, p-Syk, p-PI3-K, IP3, PLC-gamma, cAMP and Ca(+2) influx, which are known to play a critical role in TLR mediated inflammation. Earlier we found that toxic effects of BA in spleen were mediated by oxidative stress which was partially neutralized by NAC exposure. Hereby, we report that NAC treatment in conjunction with BA attenuated the expression of BA induced TLR4, as well as the inflammatory markers such as COX2 and p-NFkB in macrophages. These findings demonstrated the critical role of TLRs in the regulation of the BA-induced inflammation.
Aryl Hydrocarbon Receptor Activation Contributes to Benzanthrone-Induced Hyperpigmentation via Modulation of Melanogenic Signaling Pathways.[Pubmed:28029781]
Chem Res Toxicol. 2017 Feb 20;30(2):625-634.
Benzanthrone (BA), an oxidized polycyclic aromatic hydrocarbon (PAH), has been found to be a potential health threat to occupational workers involved in dye manufacturing factories. It has been observed that occupational workers become exposed to BA either during manufacturing, pulverization, or storage and developed various kinds of skin diseases like contact dermatitis, itching, erythema, roughness, and foremost, hyperpigmentation. It has been shown that some environmental organic pollutants (POPs) like dioxins, furans, and polychlorinated biphenyls (PCBs) may act as ligands for the aryl hydrocarbon receptor (AhR) and regulate hyperpigmentation. Here, we hypothesized that BA may also act as a ligand for AhR and possibly regulate the melanogenic pathway to induced hyperpigmentation. Our computation results indicate that BA has a high binding affinity toward AhR for the initiation of melanogenic signaling. Following the in silico predictions, we used primary mouse melanocytes (PMMs) and confirmed that exposure to BA (5, 10, and 25 muM) resulted in an increase in AhR expression, tyrosinase activity, and melanin synthesis. Moreover, to study the physiological relevance of these findings, C57BL/6 mice were topically exposed to BA, and enhanced pigmentation and melanin synthesis were observed. Furthermore, the study was extended to assess the mechanistic aspects involved in BA-induced hyperpigmentation in PMMs as well as in mouse skin. Our results suggest that BA exposure initiates AhR signaling and increases tyrosinase enzyme activity and melanin synthesis. Moreover, the genes that regulate the melanin synthesis, such as TRP-1, TRP-2 and the transcription factor MITF, were also found to be increased. Thus, altogether, we suggest that BA-AhR interactions are critical for BA-induced hyperpigmentation.
Exposure and size distribution of nitrated and oxygenated polycyclic aromatic hydrocarbons among the population using different household fuels.[Pubmed:27400906]
Environ Pollut. 2016 Sep;216:935-942.
Polycyclic aromatic hydrocarbons (PAHs) derivatives like nitrated and oxygenated PAHs are of growing concerns because of considerably higher toxicity and important roles during atmospheric chemical reactions. Residential solid fuel combustion is likely to be one large primary source of these pollutants in developing countries. In this study, inhalation exposure to nitrated and oxygenated PAH derivatives was evaluated among rural residents using carried samplers. The exposure levels of individual nitrated PAHs ranged from 4.04 (9-nitrated phenanthrene) to 89.8 (9-nitrated anthracene) pg/m(3), and of oxy-PAHs were 0.570 (benzo[a]anthracene-7, 12-dione) to 7.99 (Benzanthrone) ng/m(3), generally higher in wood user than that in anthracite user. A majority of derivatives in particle presented in PM2.5 (80% for nitrated naphthalene and over 90% for other targets) and even fine PM1.0. Mass fractions of PAH derivatives in fine and ultra-fine particles were significantly higher than the fractions of corresponding parent PAHs, indicating more adverse health outcomes induced by these derivatives. The inhalation exposure levels for residents adopting wood gasifier burners was significantly lower than the documented results for those burning wood in typical built-in brick stoves, and comparable to those using LPG and electricity, which provided vital information for clean stove development and intervention programs.


