2,2'-Bicinchoninic acidCAS# 1245-13-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1245-13-2 | SDF | Download SDF |
| PubChem ID | 71068 | Appearance | Powder |
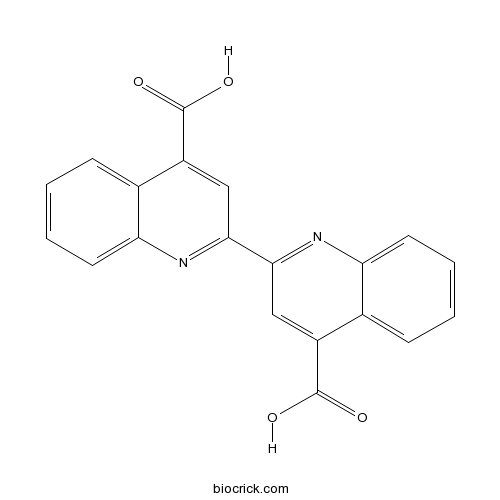
| Formula | C20H12N2O4 | M.Wt | 344.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(4-carboxyquinolin-2-yl)quinoline-4-carboxylic acid | ||
| SMILES | C1=CC=C2C(=C1)C(=CC(=N2)C3=NC4=CC=CC=C4C(=C3)C(=O)O)C(=O)O | ||
| Standard InChIKey | AFYNADDZULBEJA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H12N2O4/c23-19(24)13-9-17(21-15-7-3-1-5-11(13)15)18-10-14(20(25)26)12-6-2-4-8-16(12)22-18/h1-10H,(H,23,24)(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,2'-Bicinchoninic acid Dilution Calculator

2,2'-Bicinchoninic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9044 mL | 14.5222 mL | 29.0444 mL | 58.0889 mL | 72.6111 mL |
| 5 mM | 0.5809 mL | 2.9044 mL | 5.8089 mL | 11.6178 mL | 14.5222 mL |
| 10 mM | 0.2904 mL | 1.4522 mL | 2.9044 mL | 5.8089 mL | 7.2611 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5809 mL | 1.1618 mL | 1.4522 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5809 mL | 0.7261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 16R-sitsirikine
Catalog No.:BCN3492
CAS No.:1245-00-7
- VU 0364739 hydrochloride
Catalog No.:BCC7875
CAS No.:1244640-48-9
- CGS 21680 HCl
Catalog No.:BCC4316
CAS No.:124431-80-7
- Cannabidiolic acid
Catalog No.:BCN6127
CAS No.:1244-58-2
- MG 149
Catalog No.:BCC5149
CAS No.:1243583-85-8
- 9-(1H-Benzotriazol-1-ylmethyl)-9H-carbazole
Catalog No.:BCC8792
CAS No.:124337-34-4
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Paucinervin A
Catalog No.:BCN7308
CAS No.:1243249-16-2
- LGK-974
Catalog No.:BCC5103
CAS No.:1243244-14-5
- Wnt-C59
Catalog No.:BCC3965
CAS No.:1243243-89-1
- RN486
Catalog No.:BCC3921
CAS No.:1242156-23-5
- 12-Ursene-3,16,22-triol
Catalog No.:BCN6126
CAS No.:1242085-06-8
- Retusin
Catalog No.:BCN7794
CAS No.:1245-15-4
- 16-Oxoalisol A
Catalog No.:BCN3460
CAS No.:124515-98-6
- PI4KIII beta inhibitor 3
Catalog No.:BCC1310
CAS No.:1245319-54-3
- Glucocorticoid receptor agonist
Catalog No.:BCC1596
CAS No.:1245526-82-2
- NVP-BGT226
Catalog No.:BCC3827
CAS No.:1245537-68-1
- GR 79236
Catalog No.:BCC7215
CAS No.:124555-18-6
- ent-Labda-8(17),13Z-diene-15,16,19-triol 19-O-glucoside
Catalog No.:BCN1598
CAS No.:1245636-01-4
- Brain natriuretic peptide (1-32) (human)
Catalog No.:BCC6034
CAS No.:124584-08-3
- Daidzin 6'-O-malonate
Catalog No.:BCN8245
CAS No.:124590-31-4
- TC-A 2317 hydrochloride
Catalog No.:BCC2418
CAS No.:1245907-03-2
- 2-Methyl-4-(2-methylbutyryl)phloroglucinol
Catalog No.:BCN7175
CAS No.:124598-11-4
- 6-O-Benzoylphlorigidoside B
Catalog No.:BCN6128
CAS No.:1246012-24-7
Rapid determination of trace copper in animal feed based on micro-plate colorimetric reaction and statistical partitioning correction.[Pubmed:27979108]
Food Chem. 2017 Apr 15;221:1406-1414.
The objective of this study was to develop a micro-plate based colorimetric assay for rapid and high-throughput detection of copper in animal feed. Copper ion in animal feed was extracted by trichloroacetic acid solution and reduced to cuprous ion by hydroxylamine. The cuprous ion can chelate with 2,2'-bicinchoninic acid to form a Cu-BCA complex which was detected with high sensitivity by micro-plate reader at 354nm. The whole assay procedure can be completed within 20min. To eliminate matrix interference, a statistical partitioning correction approach was proposed, which makes the detection of copper in complex samples possible. The limit of detection was 0.035mug/mL and the detection range was 0.1-10mug/mL of copper in buffer solution. Actual sample analysis indicated that this colorimetric assay produced results consistent with atomic absorption spectrometry analysis. These results demonstrated that the developed assay can be used for rapid determination of copper in animal feed.
Robust affinity standards for Cu(I) biochemistry.[Pubmed:24298878]
J Am Chem Soc. 2013 Dec 11;135(49):18549-59.
The measurement of reliable Cu(I) protein binding affinities requires competing reference ligands with similar binding strengths; however, the literature on such reference ligands is not only sparse but often conflicting. To address this deficiency, we have created and characterized a series of water-soluble monovalent copper ligands, MCL-1, MCL-2, and MCL-3, that form well-defined, air-stable, and colorless complexes with Cu(I) in aqueous solution. X-ray structural data, electrochemical measurements, and an extensive network of equilibrium titrations showed that all three ligands form discrete Cu(I) complexes with 1:1 stoichiometry and are capable of buffering Cu(I) concentrations between 10(-10) and 10(-17) M. As most Cu(I) protein affinities have been obtained from competition experiments with bathocuproine disulfonate or 2,2'-bicinchoninic acid, we further calibrated their Cu(I) stability constants against the MCL series. To demonstrate the application of these reagents, we determined the Cu(I) binding affinity of CusF (log K = 14.3 +/- 0.1), a periplasmic metalloprotein required for the detoxification of elevated copper levels in Escherichia coli . Altogether, this interconnected set of affinity standards establishes a reliable foundation that will facilitate the precise determination of Cu(I) binding affinities of proteins and small-molecule ligands.
Determination of microquantities of thiosulfate by kinetic method.[Pubmed:18967320]
Talanta. 1998 Sep;47(1):213-21.
A new redox indicator reaction between Cu(2+), Mn(2+) and S(2)O(3)(2-) in the presence of 2,2'-bicinchoninic acid is investigated. Optimum reaction conditions are determined. The influence of nonaqueous solvents on the reaction rate is studied. It is shown that in the presence of acetone the S(2)O(3)(2-) determination limit is lowered 0.005 mug ml(-1). Random errors lie within the limits allowable at the determination of microconcentrations. To raise the sensitivity of the determination of S(2)O(3)(2-) in some inorganic salts (KCl, KNO(3), CsNO(3)), this impurity was concentrated by low-temperature directed crystallisation. Metrologic characteristics of the developed technique are presented.
Measurement of protein in natural rubber latex.[Pubmed:7485983]
Anal Biochem. 1995 Aug 10;229(2):278-81.
Latex from the Brazilian rubber tree (Hevea brasiliensis) is the source of virtually all commercial natural rubber (cis-1,4-polyisoprene). Products made from natural rubber latex include gloves, condoms, and hundreds of different medical devices. However, recent reports of widespread life-threatening latex-associated Type I allergies have focused attention on latex proteins as serious allergens. In this paper, we describe a method that permits accurate and reproducible determination of protein in latex and that includes a procedure for solubilizing latex proteins and removing rubber. Also, we show that interfering substances in latex can be removed by precipitating proteins with sodium deoxycholate and trichloroacetic acid, and that latex proteins can be recovered and quantified with 2,2'-bicinchoninic acid.
A quantitative test for copper using bicinchoninic acid.[Pubmed:7785783]
Anal Biochem. 1995 Mar 20;226(1):80-4.
We describe a direct colorimetric assay for copper in serum and biological samples using 2,2'-bicinchoninic acid (BCA), a common reagent in most laboratories. BCA offers the advantage of being highly sensitive and specific for Cu(I) which rapidly forms an intense purple complex in the presence of BCA. The complex has peak absorbances at 562 and 354.5 nm with molar absorptivities of 7.7 x 10(3) liter mol-1 cm-1 and 4.6 x 10(4) liter mol-1 cm-1, respectively. Interference by other metal ions, pH, and detergents is minimal and the results correlate strongly with atomic absorption spectrophotometry.
Colorimetric determination of reactive solid-supported primary and secondary amino groups.[Pubmed:8031990]
Biomaterials. 1994 Mar;15(4):289-97.
A simple and sensitive method for the quantitative determination of solid-supported primary and/or secondary amino groups using commercially available reagents is described. The solid supports are treated in an aqueous environment with either 2-iminothiolane (ITL) or sulpho-succinimidyl-3-(4-hydroxyphenyl)propionate (sulpho-SHPP), which introduce one sulphydryl or one hydroxyphenyl group per amino group reacted, respectively. These groups are capable of reducing Cu2+ to Cu+ in alkaline medium. Thus, after removal of the excess reagents through washing, subsequent incubation of the solids with 2,2'-bicinchoninic acid (BCA) copper protein reagent results in production of Cu+ in the solution, which forms a chelate complex with BCA absorbing at 562 nm. The quantitation of the groups introduced on the surfaces, and therefore of the reacted amino groups, is carried out through standard curves of cysteine solutions for ITL, or tyrosine solutions for sulpho-SHPP-treated solids. Using ITL, only the primary amino groups are determined, whereas sulpho-SHPP provided the primary and secondary reactive amino groups. The method is versatile and can be used for the estimation of amino groups onto several biomedical solid matrices, and should provide useful information for the covalent immobilization of ligands (e.g. drugs, antibodies).
Drug interference in the Bradford and 2,2'-bicinchoninic acid protein assays.[Pubmed:1724722]
Anal Biochem. 1991 Nov 1;198(2):352-4.
The interference of a range of drugs and related substances has been investigated in the Bradford Coomassie brilliant blue (CBB) protein dye-binding assay and the 2,2'-bicinchoninic acid (BCA) protein assays. Chlorpromazine was the only substance to interfere in the CBB assay but the interference was slight. In contrast, the BCA reagent interacted strongly with chlorpromazine, the penicillins, vitamin C, and paracetamol and the mode of interference varied with the test substance. The chlorpromazine produced turbidity and an atypical color. The penicillins show a slow but normal color response while vitamin C and paracetamol gave an immediate and intense response.
Potential interference of hydrogen peroxide in the 2,2'-bicinchoninic acid protein assay.[Pubmed:2048723]
Anal Biochem. 1991 Jan;192(1):212-4.
At a concentration of 20-800 nmol/0.1 ml hydrogen peroxide instantly reacts with a 2,2'-bicinchoninic acid copper color reagent. It also reacts with a reformulated reagent at pH 7 but the color develops less rapidly. While the effect may interfere with protein estimations at alkaline pH, the effect of pH 7 may be used to determine the possible extent of hydrogen peroxide interference after treatment with catalase.
Quantitative benedict test using bicinchoninic acid.[Pubmed:2513739]
Anal Biochem. 1989 Oct;182(1):54-7.
Cupric ion (Cu2+), in complex form, functions as a selective oxidizing agent for a variety of compounds in the qualitative Benedict test. Cupric ion is reduced to cuprous ion (Cu+) in the reaction. We found that the cuprous ion formed in this type of redox reaction could be detected and quantified using 2,2'-bicinchoninic acid. This reagent produced an intense purple complex with cuprous ion. The color development in this modified Benedict test was dependent on pH, temperature, and time. The reaction is insensitive to ethanol and sodium dodecyl sulfate. This improved method of the Benedict test has enhanced sensitivity and makes the quantitation of compounds possible. This method should be useful for studies of Benedict-positive compounds which are available only in small amounts.


