Glucocorticoid receptor agonistCAS# 1245526-82-2 |
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- Beclomethasone dipropionate
Catalog No.:BCC4257
CAS No.:5534-09-8
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Mifepristone
Catalog No.:BCC4486
CAS No.:84371-65-3
- Betamethasone hydrochloride
Catalog No.:BCC4256
CAS No.:956901-32-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1245526-82-2 | SDF | Download SDF |
| PubChem ID | 46937290 | Appearance | Powder |
| Formula | C20H20F4N2O2 | M.Wt | 396.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (252.28 mM) *"≥" means soluble, but saturation unknown. | ||
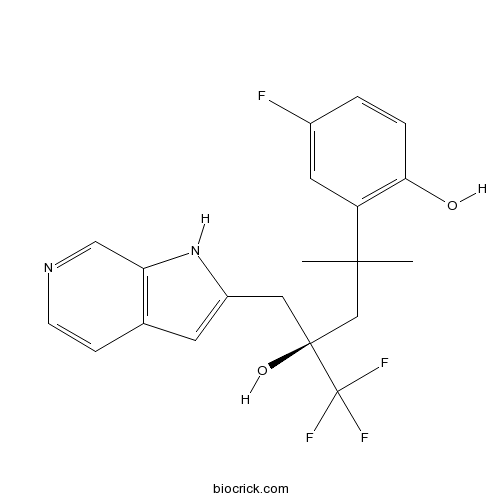
| Chemical Name | 4-fluoro-2-[(4R)-5,5,5-trifluoro-4-hydroxy-2-methyl-4-(1H-pyrrolo[2,3-c]pyridin-2-ylmethyl)pentan-2-yl]phenol | ||
| SMILES | CC(C)(CC(CC1=CC2=C(N1)C=NC=C2)(C(F)(F)F)O)C3=C(C=CC(=C3)F)O | ||
| Standard InChIKey | JFUAWXPBHXKZGA-IBGZPJMESA-N | ||
| Standard InChI | InChI=1S/C20H20F4N2O2/c1-18(2,15-8-13(21)3-4-17(15)27)11-19(28,20(22,23)24)9-14-7-12-5-6-25-10-16(12)26-14/h3-8,10,26-28H,9,11H2,1-2H3/t19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucocorticoid receptor agonist Dilution Calculator

Glucocorticoid receptor agonist Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5228 mL | 12.6142 mL | 25.2283 mL | 50.4566 mL | 63.0708 mL |
| 5 mM | 0.5046 mL | 2.5228 mL | 5.0457 mL | 10.0913 mL | 12.6142 mL |
| 10 mM | 0.2523 mL | 1.2614 mL | 2.5228 mL | 5.0457 mL | 6.3071 mL |
| 50 mM | 0.0505 mL | 0.2523 mL | 0.5046 mL | 1.0091 mL | 1.2614 mL |
| 100 mM | 0.0252 mL | 0.1261 mL | 0.2523 mL | 0.5046 mL | 0.6307 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Glucocorticoid receptor agonist is a potent Glucocorticoid receptor agonist.
- PI4KIII beta inhibitor 3
Catalog No.:BCC1310
CAS No.:1245319-54-3
- 16-Oxoalisol A
Catalog No.:BCN3460
CAS No.:124515-98-6
- Retusin
Catalog No.:BCN7794
CAS No.:1245-15-4
- 2,2'-Bicinchoninic acid
Catalog No.:BCC8487
CAS No.:1245-13-2
- 16R-sitsirikine
Catalog No.:BCN3492
CAS No.:1245-00-7
- VU 0364739 hydrochloride
Catalog No.:BCC7875
CAS No.:1244640-48-9
- CGS 21680 HCl
Catalog No.:BCC4316
CAS No.:124431-80-7
- Cannabidiolic acid
Catalog No.:BCN6127
CAS No.:1244-58-2
- MG 149
Catalog No.:BCC5149
CAS No.:1243583-85-8
- 9-(1H-Benzotriazol-1-ylmethyl)-9H-carbazole
Catalog No.:BCC8792
CAS No.:124337-34-4
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Paucinervin A
Catalog No.:BCN7308
CAS No.:1243249-16-2
- NVP-BGT226
Catalog No.:BCC3827
CAS No.:1245537-68-1
- GR 79236
Catalog No.:BCC7215
CAS No.:124555-18-6
- ent-Labda-8(17),13Z-diene-15,16,19-triol 19-O-glucoside
Catalog No.:BCN1598
CAS No.:1245636-01-4
- Brain natriuretic peptide (1-32) (human)
Catalog No.:BCC6034
CAS No.:124584-08-3
- Daidzin 6'-O-malonate
Catalog No.:BCN8245
CAS No.:124590-31-4
- TC-A 2317 hydrochloride
Catalog No.:BCC2418
CAS No.:1245907-03-2
- 2-Methyl-4-(2-methylbutyryl)phloroglucinol
Catalog No.:BCN7175
CAS No.:124598-11-4
- 6-O-Benzoylphlorigidoside B
Catalog No.:BCN6128
CAS No.:1246012-24-7
- 6-O-trans-Cinnamoylphlorigidoside B
Catalog No.:BCN6129
CAS No.:1246012-25-8
- 6-O-trans-p-Coumaroylshanzhiside methyl ester
Catalog No.:BCN1597
CAS No.:1246012-26-9
- 4'-O-trans-p-Coumaroylmussaenoside
Catalog No.:BCN6130
CAS No.:1246012-27-0
- Methyl (2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenylpropionate
Catalog No.:BCN8363
CAS No.:124605-42-1
Pharmacokinetics and food-effect of fosdagrocorat (PF-04171327), a dissociated agonist of the glucocorticoid receptor, in healthy adult Caucasian and Japanese subjects.[Pubmed:27781421]
Int J Clin Pharmacol Ther. 2016 Dec;54(12):966-976.
OBJECTIVE: Fosdagrocorat (PF-04171327) is a pro-drug form of PF-00251802, a dissociated agonist of the glucocorticoid receptor, under investigation for the treatment of rheumatoid arthritis. This study investigates the pharmacokinetics (PK) of single and multiple doses of fosdagrocorat in healthy Japanese and Western volunteers, the effect of food on fosdagrocorat PK, and the effect of fosdagrocorat on bone biomarkers. METHODS: This was a phase 1, randomized, placebo-controlled, dose-escalation study. For single-escalating-dose evaluation, Japanese (n = 9) and Western (n = 9) subjects were randomized (1 : 1 : 1) to treatment sequences including 3 doses of fosdagrocorat (5, 10, or 30 mg) or placebo. For multiple-dose evaluation, Japanese subjects were randomized (3 : 1) to receive fosdagrocorat 20 mg or placebo once daily (QD) for 12 days. Subjects were aged 18 - 55 years; body mass index 17.5 - 30.5 kg/m2; total body weight > 45 kg. RESULTS: Following single doses of fosdagrocorat, the PK of PF-00251802 and its metabolite PF-04015475 were similar between Japanese (PF-00251802: mean area under the curve (AUC)inf (range across doses), 791 - 3,460 ngxh/mL; individual half-life (t1/2) 14.1 - 28.9 hours; PF-04015475: mean AUCinf, 395 - 1,740 ngxh/mL; individual t1/2 21.6 - 40.3 hours) and Western (PF-00251802: mean AUCinf, 750 - 4,150 ngxh/mL; individual t1/2 17.7 - 40.4 hours; PF-04015475: mean AUCinf, 394 - 2,160 ngxh/mL; individual t1/2 24.5 - 63.7 hours) subjects. Steady-state concentrations were reached within 9 days following multiple doses of fosdagrocorat. Food did not affect total exposure of PF-00251802 and PF-04015475. Multiple-dose administration of fosdagrocorat 20 mg QD resulted in suppression of bone formation markers and cortisol and increased bone resorption markers vs. placebo. Adverse events (AEs) were mild in severity and no serious AEs, deaths, or severe AEs were reported. CONCLUSIONS: The PK profile of fosdagrocorat was similar between Japanese and Western subjects, with little effect of food on PK parameters. Fosdagrocorat was well tolerated in both Japanese and Western subjects..
Discovery of new selective glucocorticoid receptor agonist leads.[Pubmed:28043796]
Bioorg Med Chem Lett. 2017 Feb 1;27(3):437-442.
We report on the discovery of two new lead series for the development of Glucocorticoid receptor agonists. Firstly, the discovery of tetrahydronaphthalenes led to metabolically stable and dissociated compounds. Their binding mode to the glucocorticoid receptor could be elucidated through an X-ray structure. Closer inspection into the reaction path and analyses of side products revealed a new amino alcohol series also addressing the glucocorticoid receptor and demonstrating strong anti-inflammatory activity in vitro.
Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study.[Pubmed:28328159]
Int J Rheum Dis. 2017 Aug;20(8):960-970.
AIM: To assess efficacy and safety of fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor, in rheumatoid arthritis (RA) patients. METHODS: This multicenter, double-blind, parallel-group, active- and placebo-controlled Phase 2 study (NCT00938587) randomized 86 patients (1 : 1 : 1 : 1) to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg or placebo, all with stable background methotrexate therapy. The primary outcome was change from baseline in Disease Activity Score of 28 joints (DAS28-4[C-reactive protein (CRP)]) after 2 weeks of treatment. Secondary outcomes included American College of Rheumatology (ACR) response rates, change from baseline in ACR core components and Health Assessment Questionnaire Disability Index. RESULTS: At week 2, improvements from baseline in DAS28-4(CRP) with fosdagrocorat 10 and 25 mg, prednisone 5 mg and placebo were -1.69, -2.22, -1.17 and -0.96, respectively, and were statistically significantly greater for both fosdagrocorat doses versus placebo (P < 0.05) and for fosdagrocorat 25 mg versus prednisone 5 mg (P < 0.001). The effects of fosdagrocorat on secondary outcomes were generally consistent with those observed for the primary outcome. Adverse events (AEs) were reported for eight (38%), three (14%), four (19%) and 12 (55%) patients treated with fosdagrocorat 10 and 25 mg, prednisone 5 mg and placebo, respectively. Most AEs were mild in severity. Four patients discontinued treatment due to AEs (fosdagrocorat 10 mg, n = 2; placebo, n = 2). There were no serious AEs. CONCLUSION: Fosdagrocorat 10 and 25 mg demonstrated efficacy in improving signs and symptoms in RA patients, with manageable AEs. Additional studies are needed to assess the longer-term safety and efficacy of fosdagrocorat.
The selective glucocorticoid receptor agonist mapracorat displays a favourable safety-efficacy ratio for the topical treatment of inflammatory skin diseases in dogs.[Pubmed:27425245]
Vet Dermatol. 2017 Feb;28(1):46-e11.
BACKGROUND: Mapracorat is a nonsteroidal Selective Glucocorticoid receptor agonist (SEGRA) that is presumed to have a better therapeutic index compared to classical glucocorticoids. OBJECTIVES: To compare the efficacy and safety of mapracorat with classical glucocorticoids used for the treatment of allergic skin diseases in dogs. ANIMALS: Six laboratory beagles. METHODS: The effect of mapracorat on lipopolysaccharide-induced TNFalpha secretion from canine peripheral blood derived mononuclear cells (PBMC) was tested. In vivo, mapracorat was compared to triamcinolone acetonide using a skin inflammation model. Skin fold thickness was determined after daily administration of mapracorat and triamcinolone acetonide over 14 days. RESULTS: Mapracorat concentration dependently inhibited TNFalpha secretion from activated canine PBMC with a half maximal inhibitory concentration (IC50 ) value of approximately 0.2 nmol/L. Intradermal injection of compound 48/80 (50 mug in 50 muL saline) resulted in a clear wheal and flare reaction over the 60 min observation period. Topical pre-treatment with mapracorat (0.1%) and triamcinolone acetonide (0.015%) led to significant reduction in the wheal and flare responses compared to vehicle (acetone) treated areas. However, once daily topical administration of triamcinolone acetonide significantly reduced skin fold thickness from day 8 to 14, whereas no such reduction was observed for mapracorat. CONCLUSION: These results demonstrate that mapracorat has comparable anti-inflammatory efficacy to classical steroidal glucocorticoids under these experimental settings and maintenance of skin fold thickness indicates a better safety profile compared to triamcinolone acetonide at equipotent concentrations. This profile further suggests that SEGRAs show promise in the management of inflammatory and pruritic skin diseases in dogs.


