Brain natriuretic peptide (1-32) (human)Endogenous peptide agonist at ANP receptor A (NPR1) CAS# 124584-08-3 |
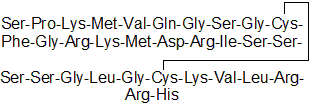
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 124584-08-3 | SDF | Download SDF |
| PubChem ID | 71308561 | Appearance | Powder |
| Formula | C143H244N50O42S4 | M.Wt | 3464.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Brain Natriuretic Peptide-32 human; BNP-32 | ||
| Solubility | H2O : ≥ 40 mg/mL (11.55 mM) *"≥" means soluble, but saturation unknown. | ||
| Sequence | SPKMVQGSGCFGRKMDRISSSSGLGCKVLR (Modifications: Disulfide bridge between 10 - 26) | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(4R,10S,16S,19S,22S,25S,28S,31S,34S,37S,40S,43S,49S,52R)-52-[[2-[[(2S)-2-[[2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-1-[(2S)-2-amino-3-hydroxypropanoyl]pyrrolidine-2-carbonyl]amino]hexanoyl]amino]-4-methylsulfanylbutanoyl]amino]-3-methylbutanoyl]amino]-5-oxopentanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-40-(4-aminobutyl)-49-benzyl-28-[(2S)-butan-2-yl]-31,43-bis(3-carbamimidamidopropyl)-34-(carboxymethyl)-16,19,22,25-tetrakis(hydroxymethyl)-10-(2-methylpropyl)-37-(2-methylsulfanylethyl)-6,9,12,15,18,21,24,27,30,33,36,39,42,45,48,51-hexadecaoxo-1,2-dithia-5,8,11,14,17,20,23,26,29,32,35,38,41,44,47,50-hexadecazacyclotripentacontane-4-carbonyl]amino]hexanoyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-(1H-imidazol-4-yl)propanoic acid | ||
| SMILES | CCC(C)C1C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NCC(=O)NC(CSSCC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCNC(=N)N)CC(=O)O)CCSC)CCCCN)CCCNC(=N)N)CC2=CC=CC=C2)NC(=O)CNC(=O)C(CO)NC(=O)CNC(=O)C(CCC(=O)N)NC(=O)C(C(C)C)NC(=O)C(CCSC)NC(=O)C(CCCCN)NC(=O)C3CCCN3C(=O)C(CO)N)C(=O)NC(CCCCN)C(=O)NC(C(C)C)C(=O)NC(CC(C)C)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC4=CNC=N4)C(=O)O)CC(C)C)CO)CO)CO)CO | ||
| Standard InChIKey | HPNRHPKXQZSDFX-OAQDCNSJSA-N | ||
| Standard InChI | InChI=1S/C143H244N50O42S4/c1-13-76(10)112-137(232)189-99(68-199)131(226)188-98(67-198)130(225)187-97(66-197)129(224)186-96(65-196)117(212)166-59-105(202)169-90(52-72(2)3)114(209)163-61-107(204)171-100(132(227)177-83(32-19-22-44-146)124(219)190-111(75(8)9)136(231)184-91(53-73(4)5)127(222)175-84(34-24-46-159-141(151)152)121(216)174-85(35-25-47-160-142(153)154)122(217)185-94(139(234)235)55-78-57-157-71-167-78)69-238-239-70-101(172-108(205)62-165-116(211)95(64-195)170-106(203)60-162-113(208)87(38-39-103(148)200)181-135(230)110(74(6)7)191-126(221)89(41-51-237-12)179-120(215)82(31-18-21-43-145)180-134(229)102-37-27-49-193(102)138(233)79(147)63-194)133(228)182-92(54-77-28-15-14-16-29-77)115(210)164-58-104(201)168-80(33-23-45-158-140(149)150)118(213)173-81(30-17-20-42-144)119(214)178-88(40-50-236-11)123(218)183-93(56-109(206)207)128(223)176-86(125(220)192-112)36-26-48-161-143(155)156/h14-16,28-29,57,71-76,79-102,110-112,194-199H,13,17-27,30-56,58-70,144-147H2,1-12H3,(H2,148,200)(H,157,167)(H,162,208)(H,163,209)(H,164,210)(H,165,211)(H,166,212)(H,168,201)(H,169,202)(H,170,203)(H,171,204)(H,172,205)(H,173,213)(H,174,216)(H,175,222)(H,176,223)(H,177,227)(H,178,214)(H,179,215)(H,180,229)(H,181,230)(H,182,228)(H,183,218)(H,184,231)(H,185,217)(H,186,224)(H,187,225)(H,188,226)(H,189,232)(H,190,219)(H,191,221)(H,192,220)(H,206,207)(H,234,235)(H4,149,150,158)(H4,151,152,159)(H4,153,154,160)(H4,155,156,161)/t76-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,110-,111-,112-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous peptide secreted from cardiac ventricles in response to volume increase and pressure overload that acts as an agonist at atrial natriuretic peptide (ANP) receptor A (NRP1). Decreases de novo collagen synthesis and increases MMP gene expression in vitro. Exhibits natriuretic, vasodilatory and lusitropic activity and inhibits the sympathetic and renin-angiotensin-aldosterone systems in vivo. |

Brain natriuretic peptide (1-32) (human) Dilution Calculator

Brain natriuretic peptide (1-32) (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nesiritide is an agonist of natriuretic peptide receptors (NPRs), with Kd values of 7.3 and 13 pM for NPR-A and NPR-C, respectively. Sequence: Ser-Pro-Lys-Met-Val-Gln-Gly-Ser-Gly-Cys-Phe-Gly-Arg-Lys-Met-Asp-Arg-Ile-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys-Lys-Val-Leu-Arg-Arg-His.
In Vitro:Nesiritide is an agonist of natriuretic peptide receptor (NPR), with Kd values of 7.3 and 13 pM for NPR-A and NPR-C, respectively[1]. ProBNP1-108 stimulates guanylyl cyclase-A (GC-A) to near-maximum activities but is 13-fold less potent than Nesiritide (BNP1-32). ProBNP1-108 binds human GC-A 35-fold less tightly than Nesiritide. Neither proBNP1–108 nor Nesiritide activates GC-B. The natriuretic peptide clearance receptor binds proBNP1-108 3-fold less tightly than Nesiritide. The half time for degradation of proBNP1-108 by human kidney membranes is 2.7-fold longer than for Nesiritide, and the time required for complete degradation is 6-fold longer. Nesiritide and proBNP1-108 are best fitted by first- and second-order exponential decay models, respectively[2].
References:
[1]. Koller KJ, et al. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992 Oct;86(4):1081-8.
[2]. Dickey DM, et al. ProBNP(1-108) is resistant to degradation and activates guanylyl cyclase-A with reduced potency. Clin Chem. 2011 Sep;57(9):1272-8.
- ent-Labda-8(17),13Z-diene-15,16,19-triol 19-O-glucoside
Catalog No.:BCN1598
CAS No.:1245636-01-4
- GR 79236
Catalog No.:BCC7215
CAS No.:124555-18-6
- NVP-BGT226
Catalog No.:BCC3827
CAS No.:1245537-68-1
- Glucocorticoid receptor agonist
Catalog No.:BCC1596
CAS No.:1245526-82-2
- PI4KIII beta inhibitor 3
Catalog No.:BCC1310
CAS No.:1245319-54-3
- 16-Oxoalisol A
Catalog No.:BCN3460
CAS No.:124515-98-6
- Retusin
Catalog No.:BCN7794
CAS No.:1245-15-4
- 2,2'-Bicinchoninic acid
Catalog No.:BCC8487
CAS No.:1245-13-2
- 16R-sitsirikine
Catalog No.:BCN3492
CAS No.:1245-00-7
- VU 0364739 hydrochloride
Catalog No.:BCC7875
CAS No.:1244640-48-9
- CGS 21680 HCl
Catalog No.:BCC4316
CAS No.:124431-80-7
- Cannabidiolic acid
Catalog No.:BCN6127
CAS No.:1244-58-2
- Daidzin 6'-O-malonate
Catalog No.:BCN8245
CAS No.:124590-31-4
- TC-A 2317 hydrochloride
Catalog No.:BCC2418
CAS No.:1245907-03-2
- 2-Methyl-4-(2-methylbutyryl)phloroglucinol
Catalog No.:BCN7175
CAS No.:124598-11-4
- 6-O-Benzoylphlorigidoside B
Catalog No.:BCN6128
CAS No.:1246012-24-7
- 6-O-trans-Cinnamoylphlorigidoside B
Catalog No.:BCN6129
CAS No.:1246012-25-8
- 6-O-trans-p-Coumaroylshanzhiside methyl ester
Catalog No.:BCN1597
CAS No.:1246012-26-9
- 4'-O-trans-p-Coumaroylmussaenoside
Catalog No.:BCN6130
CAS No.:1246012-27-0
- Methyl (2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenylpropionate
Catalog No.:BCN8363
CAS No.:124605-42-1
- VU 0364439
Catalog No.:BCC1239
CAS No.:1246086-78-1
- C3bot (154-182)
Catalog No.:BCC6117
CAS No.:1246280-79-4
- Officinaruminane B
Catalog No.:BCN3594
CAS No.:1246282-67-6
- (-)-Dihydroguaiaretic acid
Catalog No.:BCN8002
CAS No.:124649-78-1
The effects of sucrose on stability of human brain natriuretic peptide [hBNP (1-32)] and human parathyroid hormone [hPTH (1-34)].[Pubmed:16316450]
J Pept Res. 2005 Dec;66(6):348-56.
Although the effect of sucrose on the physical stability of proteins has been well documented, its impact on their chemical stability is largely unknown. The aim of this study was to investigate the potential effects of sucrose on the structural conformation of human brain natriuretic peptide [hBNP (1-32)] and the synthetic human parathyroid hormone [hPTH (1-34)], and link these effects to chemical degradation pathways of these peptides. The stability of hBNP (1-32) and hPTH (1-34) was studied at pH 5.5. Aggregation was monitored using size exclusion high-performance liquid chromatography (SE-HPLC), whereas oxidation and deamidation products were measured by reversed phase (RP) HPLC. Fourier transform infrared (FT-IR) spectroscopy was used to study the peptides' conformation. Sucrose retarded aggregation, deamidation, and oxidation of hBNP (1-32) and hPTH (1-34), with a maximum effect at relatively high concentrations (as much as 1 m). FT-IR spectroscopy indicated that sucrose maintained the native conformation of hBNP (1-32) and induced small conformation changes in the hPTH (1-34) structure. Sucrose enhanced the stability of hBNP (1-32) and hPTH (1-34) in liquid formulations. The stabilizing effect of sucrose was due to a large extent to retardation of oxidation and deamidation of hBNP (1-32) and hPTH (1-34).
Immunoreactivity and guanosine 3',5'-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide.[Pubmed:17372040]
Hypertension. 2007 May;49(5):1114-9.
Recent studies support the speculation that different molecular forms of the cardiac hormone BNP with differential biological activity may circulate in heart failure and be detected by conventional assays. In the current study we determined the ability of 3 widely used conventional assays to detect these different forms thought to circulate in heart failure. We also evaluated the ability of pro-BNP (1-108), N-terminal peptide (NT)-pro-BNP (1-76), and BNP 3-32, the latter a cleavage product of BNP 1-32 by dipeptidyl peptidase IV, on an equimolar basis to activate cGMP in cultured cardiac fibroblasts and cardiomyocytes compared with the biologically active mature BNP 1-32. Specifically, we observed that the Roche NT-pro-BNP assay detected both NT-pro-BNP 1-76 and pro-BNP 1-108 and that Biosite Triage and Shionogi detected both mature BNP 1-32 and the shortened BNP 3-32. Moreover, in cultured cardiac fibroblasts and cardiomyocytes, BNP 1-32 (10(-6) mol/L) activated cGMP. BNP 3-32 demonstrated a similar cGMP activating property in both cardiac cell types. In contrast, the cGMP response to pro-BNP 1-108 and NT-pro-BNP 1-76 was not significantly greater than no treatment alone. We conclude that widely used commercial assays for NT-pro-BNP 1-76 and BNP 1-32 cannot differentiate among pro-, processed, or degraded forms and, thus, may not thoroughly identify circulating BNP forms in heart failure patients. These findings also demonstrate differential cGMP activating properties of BNP forms and, importantly, that pro-BNP 1-108 and NT-pro-BNP 1-76 have reduced cGMP activity in vitro that may have biological relevance to human heart failure.
Natriuretic peptides, but not nitric oxide donors, elevate levels of cytosolic guanosine 3',5'-cyclic monophosphate in ependymal cells ex vivo.[Pubmed:16278044]
Neurosci Lett. 2006 Jan 16;392(3):187-92.
Atrial natriuretic peptide-(1-28) (ANP), brain natriuretic peptide-(1-32) (BNP) and C-Type natriuretic polypeptide (CNP) occur in the brain, are concentrated in the anteroventral area of the third cerebral ventricle and participate in the regulation of body fluid homeostasis. The ventricles of the mammalian brain are lined by a continuous monolayered epithelium of polyciliated ependymal cells. In the adult rat, the ependymocytes continue to express the intermediate filament vimentin, but do not contain glial fibrillary acidic protein. Ependymal functions are poorly understood, but may extend to osmoregulation and volume sensing. Ependymal cells possess receptors for the natriuretic peptides, and in cell culture respond to them with an increase in their cyclic GMP content. In this study, a cyclic GMP-specific antibody was employed together with an ex vivo brain slice system to assess the ependymal response to ANP, BNP and CNP under close to life-like conditions. While ANP in concentrations of 0.1 nM and 1 nM had no effect, at concentrations of 10nM and 100 nM it increased ependymal cyclic GMP levels in a concentration-dependent manner. The other natriuretic peptides BNP, and CNP, also increased the cyclic GMP content of ependymocytes, while nitric oxide (NO) donors had no effect. However, in contrast to the natriuretic peptides, the NO donors elevated the level of cyclic GMP in the brain parenchyma below the ependymal layer.
Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases.[Pubmed:12480813]
Circ Res. 2002 Dec 13;91(12):1127-34.
Cardiac fibroblasts (CFs) produce extracellular matrix proteins and participate in the remodeling of the heart. It is unknown if brain natriuretic peptide (BNP) is synthesized by CFs and if BNP participates in the regulation of extracellular matrix turnover. In this study, we examined the production of BNP in adult canine CFs and the role of BNP and its signaling system on collagen synthesis and on the activation of matrix metalloproteinases (MMPs). BNP mRNA was detected in CFs, and a specific radioimmunoassay demonstrated that BNP(1-32) was secreted into the media at a rate of 11.2+/-1.0 pg/10(5) cells per 48 hours (mean+/-SEM). The amount of BNP secretion was significantly (P<0.01) augmented by 10(-7) mol/L tumor necrosis factor-alpha in a time-dependent manner. BNP significantly (P<0.01) inhibited de novo collagen synthesis as assessed by [3H]proline incorporation, whereas zymographic MMP-2 (gelatinase) abundance was significantly (P<0.05) stimulated by BNP between 10(-7) and 10(-6) mol/L. In addition, protein expression of MMP-1, -2, and -3 and membranous type-1 MMP was significantly increased by 10(-6) mol/L BNP. The cGMP analogue 8-bromo-cGMP (10(-4) mol/L) mimicked the BNP effect, whereas inhibition of protein kinase G by KT5823 (10(-6) mol/L) significantly (P<0.05) attenuated BNP-induced zymographic MMP-2 abundance. In summary, this study reports that BNP is present in cultured CFs and that BNP decreases collagen synthesis and increases MMPs via cGMP-protein kinase G signaling. These in vitro findings support a role for BNP as a regulator of myocardial structure via control of cardiac fibroblast function.


