PI4KIII beta inhibitor 3PI4KIII beta inhibitor CAS# 1245319-54-3 |
- PF-04691502
Catalog No.:BCC3837
CAS No.:1013101-36-4
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- PIK-93
Catalog No.:BCC2519
CAS No.:593960-11-3
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1245319-54-3 | SDF | Download SDF |
| PubChem ID | 46916382 | Appearance | Powder |
| Formula | C22H22N8OS | M.Wt | 446.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
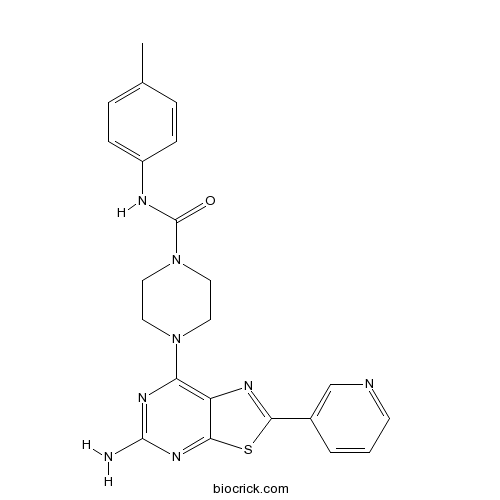
| Chemical Name | 4-(5-amino-2-pyridin-3-yl-[1,3]thiazolo[5,4-d]pyrimidin-7-yl)-N-(4-methylphenyl)piperazine-1-carboxamide | ||
| SMILES | CC1=CC=C(C=C1)NC(=O)N2CCN(CC2)C3=C4C(=NC(=N3)N)SC(=N4)C5=CN=CC=C5 | ||
| Standard InChIKey | UWTRKIJAGTTXNM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H22N8OS/c1-14-4-6-16(7-5-14)25-22(31)30-11-9-29(10-12-30)18-17-20(28-21(23)27-18)32-19(26-17)15-3-2-8-24-13-15/h2-8,13H,9-12H2,1H3,(H,25,31)(H2,23,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PI4KIII beta inhibitor 3 is a novel and high effective ΡΙ4ΚΙΙΙβ inhibitor with IC50 of 5.7 nM.In Vitro:PI4KIII beta inhibitor 3 is a PI4KIII inhibitor extracted from patent WO/2013034738 A1, the compound of formula 3, has an IC50 of 5.7 nM. PI4KIII beta inhibitor 3 exerts significant immunosuppressive activity, with IC50 value of 3 nM in the mixed lymphocyte reaction (MLR) assay. PI4KIII beta inhibitor 3 inhibits IL2 and IFNy secretion with IC50 values of less than InM in each case. Thus, PI4KIII beta inhibitor 3 is shown to be as effective at inhibiting IL2 and IFNy secretion as conventional immunosuppressants such as cyclosporine A. IC50 on IFNy and IL-2 release of Cyclosporine A are 2nM and less than 1 nM respectively[1].In Vivo:PI4KIII beta inhibitor 3 (40 mg/kg per day, n=12) is able to delay the onset of arthritic symptoms and also to decrease symptom severity in a preventive model of arthritis compared to a vehicle control (MC 1%, n=12). PI4KIII beta inhibitor 3 reduces the anti-CII IgG titre and histological scores in the collagen-induced arthritis mouse model. Oral administration of PI4KIII beta inhibitor 3 results in prolonged graft survival in 3 out of 6 grafts in each group at day 30. Several grafts continued beating after withdrawal of the treatment (up to 60 days), indicating the induction of a certain type of graft tolerance. To evaluate the operational tolerance phenotype, animals with functional graft at day 60 are challenged with a second graft from the same donor strain or from a third party. No treatment is applied. The second grafts from the third party are rejected at day 8 (n=2) whereas second grafts from the same donor strain are functional for more than 90 days (n=2)[1]. References: | |||||

PI4KIII beta inhibitor 3 Dilution Calculator

PI4KIII beta inhibitor 3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2395 mL | 11.1975 mL | 22.3949 mL | 44.7898 mL | 55.9873 mL |
| 5 mM | 0.4479 mL | 2.2395 mL | 4.479 mL | 8.958 mL | 11.1975 mL |
| 10 mM | 0.2239 mL | 1.1197 mL | 2.2395 mL | 4.479 mL | 5.5987 mL |
| 50 mM | 0.0448 mL | 0.2239 mL | 0.4479 mL | 0.8958 mL | 1.1197 mL |
| 100 mM | 0.0224 mL | 0.112 mL | 0.2239 mL | 0.4479 mL | 0.5599 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 5.7 nM[1] PI4KIII beta inhibitor 3, 4-(5-amino-2-(pyridin-3-yl)thiazolo[5,4-d]pyrimidin-7-yl)-N-p-tolylpiperazine-1-carboxamide, is a novel and high effective PI4KIII beta inhibitor. in vitro: The most effective test compound, the compound of formula 3 (PI4KIII beta inhibitor 3), inhibited IL2 and IFNy secretion with IC50 values of less than 1 nM in each case. Thus, the compound of formula (3) was shown to be as effective at inhibiting IL2 and IFNy secretion as conventional immunosuppressants such as cyclosporine A. IC50 on IFNy and IL-2 release of Cyclosporine A are 2nM and less than 1 nM respectively [1]. in vivo: Twelve animals received daily treatment with vehicle (1%methylcellulose), twelve others received PI4KIII beta inhibitor 3 at 40 mg/kg/d in 1% methylcellulose [1]. Clinical trial: N/A
- 16-Oxoalisol A
Catalog No.:BCN3460
CAS No.:124515-98-6
- Retusin
Catalog No.:BCN7794
CAS No.:1245-15-4
- 2,2'-Bicinchoninic acid
Catalog No.:BCC8487
CAS No.:1245-13-2
- 16R-sitsirikine
Catalog No.:BCN3492
CAS No.:1245-00-7
- VU 0364739 hydrochloride
Catalog No.:BCC7875
CAS No.:1244640-48-9
- CGS 21680 HCl
Catalog No.:BCC4316
CAS No.:124431-80-7
- Cannabidiolic acid
Catalog No.:BCN6127
CAS No.:1244-58-2
- MG 149
Catalog No.:BCC5149
CAS No.:1243583-85-8
- 9-(1H-Benzotriazol-1-ylmethyl)-9H-carbazole
Catalog No.:BCC8792
CAS No.:124337-34-4
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Paucinervin A
Catalog No.:BCN7308
CAS No.:1243249-16-2
- LGK-974
Catalog No.:BCC5103
CAS No.:1243244-14-5
- Glucocorticoid receptor agonist
Catalog No.:BCC1596
CAS No.:1245526-82-2
- NVP-BGT226
Catalog No.:BCC3827
CAS No.:1245537-68-1
- GR 79236
Catalog No.:BCC7215
CAS No.:124555-18-6
- ent-Labda-8(17),13Z-diene-15,16,19-triol 19-O-glucoside
Catalog No.:BCN1598
CAS No.:1245636-01-4
- Brain natriuretic peptide (1-32) (human)
Catalog No.:BCC6034
CAS No.:124584-08-3
- Daidzin 6'-O-malonate
Catalog No.:BCN8245
CAS No.:124590-31-4
- TC-A 2317 hydrochloride
Catalog No.:BCC2418
CAS No.:1245907-03-2
- 2-Methyl-4-(2-methylbutyryl)phloroglucinol
Catalog No.:BCN7175
CAS No.:124598-11-4
- 6-O-Benzoylphlorigidoside B
Catalog No.:BCN6128
CAS No.:1246012-24-7
- 6-O-trans-Cinnamoylphlorigidoside B
Catalog No.:BCN6129
CAS No.:1246012-25-8
- 6-O-trans-p-Coumaroylshanzhiside methyl ester
Catalog No.:BCN1597
CAS No.:1246012-26-9
- 4'-O-trans-p-Coumaroylmussaenoside
Catalog No.:BCN6130
CAS No.:1246012-27-0
Interplay of genes in plant-pathogen interactions: In planta expression and docking studies of a beta 1,3 glucanase gene from Piper colubrinum and a glucanase inhibitor gene from Phytophthora capsici.[Pubmed:27924129]
Physiol Mol Biol Plants. 2016 Oct;22(4):567-573.
Oomycete pathogen, Phytophthora capsici is devastating for black pepper (Piper nigrum L.) and causes foot rot disease at all stages of plant growth. Phytophthora secretes a glucanase inhibitor protein (GIP), which is capable of inhibiting defence proteins like endoglucanases. In this particular study Quantitative PCR analysis, molecular docking studies and analysis of sequences of Glucanase inhibitor protein and beta-1,3 glucanse genes were done mainly depending on the data derived from Phytophthora capsici whole genome sequencing and Piper colubrinum RNA-sequencing (RNA-Seq). Amino acid sequence length of GIP gene from P. capsici was about 353 amino acids and that of glucanase pcEGase gene from P. colubrinum was about 312 amino acids. GIP gene from P. capsici showed high level of expression at early hours of the inoculation time period and pcEGase gene showed high level of expression at 16 hpi. High level of expression of pcEGase gene at 16 hpi is an indication that the GIP gene is successfully inhibited by the glucanase protein from the plant. Moreover insilico studies gave some hint on the importance of certain sites on the surfaces of both interacting proteins that might be having a role in binding of the two proteins and subsequent reactions thereof. Insilico analysis also conclusively proved that inhibition of glucanase inhibitor protein is mainly caused by recognition of an arginine as well as an isoleucine residue during the interaction of the two proteins.
Discovery of a Phosphoinositide 3-Kinase (PI3K) beta/delta Inhibitor for the Treatment of Phosphatase and Tensin Homolog (PTEN) Deficient Tumors: Building PI3Kbeta Potency in a PI3Kdelta-Selective Template by Targeting Nonconserved Asp856.[Pubmed:28106991]
J Med Chem. 2017 Feb 23;60(4):1555-1567.
Phosphoinositide 3-kinase (PI3K) beta signaling is required to sustain cancer cell growth in which the tumor suppressor phosphatase and tensin homolog (PTEN) has been deactivated. This manuscript describes the discovery, optimization, and in vivo evaluation of a novel series of PI3Kbeta/delta inhibitors in which PI3Kbeta potency was built in a PI3Kdelta-selective template. This work led to the discovery of a highly selective PI3Kbeta/delta inhibitor displaying excellent pharmacokinetic profile and efficacy in a human PTEN-deficient LNCaP prostate carcinoma xenograft tumor model.
Discovery of the 3-Imino-1,2,4-thiadiazinane 1,1-Dioxide Derivative Verubecestat (MK-8931)-A beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibitor for the Treatment of Alzheimer's Disease.[Pubmed:27933948]
J Med Chem. 2016 Dec 8;59(23):10435-10450.
Verubecestat 3 (MK-8931), a diaryl amide-substituted 3-imino-1,2,4-thiadiazinane 1,1-dioxide derivative, is a high-affinity beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor currently undergoing Phase 3 clinical evaluation for the treatment of mild to moderate and prodromal Alzheimer's disease. Although not selective over the closely related aspartyl protease BACE2, verubecestat has high selectivity for BACE1 over other key aspartyl proteases, notably cathepsin D, and profoundly lowers CSF and brain Abeta levels in rats and nonhuman primates and CSF Abeta levels in humans. In this annotation, we describe the discovery of 3, including design, validation, and selected SAR around the novel iminothiadiazinane dioxide core as well as aspects of its preclinical and Phase 1 clinical characterization.
Na(+)/H(+) exchanger 3 inhibitor diminishes hepcidin-enhanced duodenal calcium transport in hemizygous beta-globin knockout thalassemic mice.[Pubmed:27995414]
Mol Cell Biochem. 2017 Mar;427(1-2):201-208.
Recent investigation has shown that the liver-derived iron-regulating hormone, hepcidin, can potentiate intestinal calcium absorption in hemizygous beta-globin knockout thalassemic (BKO) mice. Since the upregulation of Fe(2+) and H(+) cotransporter, divalent metal transporter (DMT)-1, has been shown to correlate with thalassemia-induced intestinal calcium absorption impairment, the inhibition of the apical Na(+)/H(+) exchanger (NHE)-3 that is essential for cytoplasmic pH regulation and transepithelial sodium absorption was hypothesized to negatively affect hepcidin action. Herein, the positive effect of hepcidin on the duodenal calcium transport was evaluated using Ussing chamber technique. The results showed that BKO mice had lower absorptive surface area and duodenal calcium transport than wild-type mice. Besides, paracellular transport of zinc in BKO mice was compromised. Hepcidin administration completely restored calcium transport. Since this hepcidin action was totally abolished by inhibitors of the basolateral calcium transporters, Na(+)/Ca(2+) exchanger (NCX1) and plasma membrane Ca(2+)-ATPase (PMCA1b), the enhanced calcium flux potentially occurred through the transcellular pathway rather than paracellular pathway. Interestingly, the selective NHE3 inhibitor, 100 nM tenapanor, markedly inhibited hepcidin-enhanced calcium transport. Accordingly, hepcidin is one of the promising therapeutic agents for calcium malabsorption in beta-thalassemia. It mainly stimulates the transcellular calcium transport across the duodenal epithelium in an NHE3-dependent manner.


