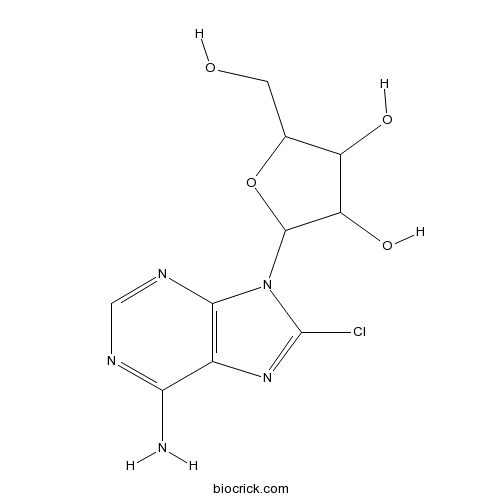
8-ChloroadenosineCytotoxic nucleoside analog; induces G2/M arrest CAS# 34408-14-5 |
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34408-14-5 | SDF | Download SDF |
| PubChem ID | 337175 | Appearance | Powder |
| Formula | C10H12ClN5O4 | M.Wt | 301.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in water with gentle warming and to 100 mM in DMSO | ||
| Chemical Name | 2-(6-amino-8-chloropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | C1=NC2=C(C(=N1)N)N=C(N2C3C(C(C(O3)CO)O)O)Cl | ||
| Standard InChIKey | MHDPPLULTMGBSI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H12ClN5O4/c11-10-15-4-7(12)13-2-14-8(4)16(10)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nucleoside analog; metabolized in vivo to 8-Chloro-ATP. Incorporates into RNA during transcription and inhibits RNA synthesis. Exhibits cytotoxicity in MM.1S, RPMI-8226 and U266 cancer cell lines; induces G2/M cell cycle arrest and mitotic catastrophe in A549 and H1299 cells. |

8-Chloroadenosine Dilution Calculator

8-Chloroadenosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3147 mL | 16.5733 mL | 33.1466 mL | 66.2932 mL | 82.8665 mL |
| 5 mM | 0.6629 mL | 3.3147 mL | 6.6293 mL | 13.2586 mL | 16.5733 mL |
| 10 mM | 0.3315 mL | 1.6573 mL | 3.3147 mL | 6.6293 mL | 8.2867 mL |
| 50 mM | 0.0663 mL | 0.3315 mL | 0.6629 mL | 1.3259 mL | 1.6573 mL |
| 100 mM | 0.0331 mL | 0.1657 mL | 0.3315 mL | 0.6629 mL | 0.8287 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-Lys(Z)-OSu
Catalog No.:BCC3418
CAS No.:34404-36-9
- H-D-Lys(Z)-OH
Catalog No.:BCC2678
CAS No.:34404-32-5
- Boc-D-Glu-OBzl
Catalog No.:BCC3394
CAS No.:34404-30-3
- Betamipron
Catalog No.:BCC8876
CAS No.:3440-28-6
- H-D-Pro-OH
Catalog No.:BCC3023
CAS No.:344-25-2
- N-Demethyl-alpha-obscurine
Catalog No.:BCN7362
CAS No.:34399-44-5
- Isovallesiachotamine
Catalog No.:BCN3549
CAS No.:34384-71-9
- Acebutolol HCl
Catalog No.:BCC4322
CAS No.:34381-68-5
- CP-673451
Catalog No.:BCC4981
CAS No.:343787-29-1
- Ginsenoside Ro
Catalog No.:BCN5937
CAS No.:34367-04-9
- Isoapetalic acid
Catalog No.:BCN5276
CAS No.:34366-34-2
- NS 3623
Catalog No.:BCC6190
CAS No.:343630-41-1
- Lathyrol
Catalog No.:BCN4963
CAS No.:34420-19-4
- 2,3-dihydrosciadopitysin
Catalog No.:BCN4034
CAS No.:34421-19-7
- Lyoniside
Catalog No.:BCN5277
CAS No.:34425-25-7
- Ikshusterol
Catalog No.:BCN5278
CAS No.:34427-61-7
- Ligularine
Catalog No.:BCN2090
CAS No.:34429-54-4
- Tirandamycin A
Catalog No.:BCN1861
CAS No.:34429-70-4
- Prudomestin
Catalog No.:BCN5279
CAS No.:3443-28-5
- Alpha-Onocerin diacetate
Catalog No.:BCN6700
CAS No.:34434-99-6
- Amycomycin
Catalog No.:BCN1824
CAS No.:344362-08-9
- Nortrachelogenin
Catalog No.:BCN5280
CAS No.:34444-37-6
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
8-Chloroadenosine Sensitivity in Renal Cell Carcinoma Is Associated with AMPK Activation and mTOR Pathway Inhibition.[Pubmed:26313261]
PLoS One. 2015 Aug 27;10(8):e0135962.
The adenosine analog 8-Chloroadenosine has been shown to deplete ATP and inhibit tumor growth in hematological malignancies as well as in lung and breast cancer cell lines. We investigated effects of 8-Chloroadenosine on clear cell (cc) renal cell carcinoma (RCC) cell lines. 8-Chloroadenosine was effective against ccRCC cell viability in vitro, with IC50 ranging from 2 muM in the most sensitive CAKI-1 to 36 muM in the most resistant RXF-393. Proteomic analysis by reverse-phase protein array revealed that 8-Chloroadenosine treatment leads to inhibition of the mTOR pathway. In time-course experiments, 8-Chloroadenosine treatment rapidly activated AMPK, measured by AMPK and ACC phosphorylation, and subsequently caused dephosphorylation of p70S6K and ribosomal protein RPS6 in the sensitive cell lines. However, in the resistant cell lines, AMPK activity and the mTOR pathway were unaffected by the treatment. We also noted that the resistant cell lines had elevated basal levels of phospho RPS6 and AKT. Inhibition of PI3K pathway enhanced the efficacy of 8-Chloroadenosine across all cell lines. Our observations indicate that 8-Chloroadenosine activity is associated with inhibition of the mTOR pathway, and that phospho RPS6 and PI3K pathway activation status may determine resistance. Among solid tumors, RCC is one of the few susceptible to mTOR inhibition. We thus infer that 8-Chloroadenosine may be effective in RCC by activating AMPK and inhibiting the mTOR pathway.
Regioselective enzymatic undecylenoylation of 8-chloroadenosine and its analogs with biomass-based 2-methyltetrahydrofuran as solvent.[Pubmed:22705510]
Bioresour Technol. 2012 Aug;118:82-8.
2-Methyltetrahydrofuran (MeTHF), a biomass-derived compound, is a promising medium for biocatalysis and organometallic reactions. The regioselective acylation of 8-Chloroadenosine (8-Cl-Ado) and its analogs was carried out in MeTHF with immobilized Penicillium expansum lipase. The lipase displayed more than twofold higher catalytic activity and much better thermostability in MeTHF than in other organic solvents and co-solvent systems. The optimum reaction medium, enzyme dosage, molar ratio of viny ester to nucleoside and reaction temperature for the enzymatic acylation of 8-Cl-Ado were MeTHF, 25 U/mL, 7.5 and 35 degrees C, respectively, under which the desirable 5'-O-undecylenoyl-8-Cl-Ado was obtained with a yield of 95% and a regioselectivity of >99% in 3 h. In addition, the lipase catalyzed regioselective undecylenoylation of other purine nucleosides, producing 5'-undecylenic acid esters with moderate to high yields (63-94%) and excellent 5'-regioselectivities (94->99%). Use of biomass-derived solvents might open up novel opportunities for sustainable and greener biocatalytic processes.
8-Chloroadenosine 3',5'-monophosphate induces cell cycle arrest and apoptosis in multiple myeloma cells through multiple mechanisms.[Pubmed:23226809]
Oncol Lett. 2012 Dec;4(6):1384-1388.
The aim of this study was to investigate the molecular mechanism of 8-Chloroadenosine 3',5'-monophosphate (8-Cl-cAMP) in the inhibition of the growth and induction of apoptosis of multiple myeloma (MM) cells. Two MM-derived cell lines, RPMI-8226 and U266, were used. Cell viability, apoptosis induction and mitochondrial transmembrane potential were determined and the expression levels of cell cycle regulatory proteins (Cdk2, cyclin E, p27 and c-myc) and p38 mitogen-activated protein kinase (MAPK) protein were detected. Following treatment with 8-Cl-cAMP, the percentage of apoptotic cells increased in a concentration- and time-dependent manner and the mitochondrial transmembrane potential collapsed to reveal typical apoptotic features. Our data further demonstrated that 8-Cl-cAMP induced progressive phosphorylation of p38 MAPK and that the expression levels of p27 proteins in the MM cells were increased whereas those of c-myc were significantly decreased. Notably, the proapoptotic effect of 8-Cl-cAMP was largely prevented by a p38 MAPK inhibitor. Furthermore, knockdown of p27 was able to decrease the 8-Cl-cAMP-induced apoptosis in the MM cells. These results indicate that 8-Cl-cAMP induced p27-dependent cell cycle arrest and apoptosis in the MM cells, which demonstrates the potential of cAMP-modulating agents for use in the treatment of MM.
Intracellular succinylation of 8-chloroadenosine and its effect on fumarate levels.[Pubmed:20064937]
J Biol Chem. 2010 Mar 12;285(11):8022-30.
8-Chloroadenosine (8-Cl-Ado) is a ribosyl nucleoside analog currently in phase I testing for the treatment of chronic lymphocytic leukemia (CLL). 8-Cl-Ado activity is dependent on adenosine kinase and requires intracellular accumulation of 8-Cl-Ado as mono-, di-, and tri-phosphates. In the current study with four mantle cell lymphoma cell lines, we report a new major metabolic pathway for 8-Cl-Ado intracellular metabolism, the formation of succinyl-8-chloro-adenosine (S-8-Cl-Ado) and its monophosphate (S-8-Cl-AMP). 8-Cl-AMP levels were highly associated with S-8-Cl-AMP levels and reached a steady-state prior to the secondary metabolites, 8-Cl-ATP and S-8-Cl-Ado. Consistent with fumarate as a required substrate for formation of succinyl-8-Cl-adenylate metabolites, the S-8-Cl-adenylate concentrations in multiple cell lines were associated with fumarate loss. The distribution of metabolites was also altered using the energy metabolism modifiers, metformin and oligomycin. The rates of succinyl-8-Cl-adenylate metabolism were enhanced by increasing the intracellular fumarate concentrations after metformin co-treatment. In addition, the S-8-Cl-AMP concentrations were increased after acute inhibition of ATP synthase by oligomycin. We conclude that 8-Cl-Ado metabolism not only affects intracellular purine metabolism; 8-Cl-Ado conversion to succinyl analogs ties its metabolism to the citric acid cycle by reduction of the fumarate pool.
ATP analog enhances the actions of a heat shock protein 90 inhibitor in multiple myeloma cells.[Pubmed:21821695]
J Pharmacol Exp Ther. 2011 Nov;339(2):545-54.
Heat shock protein (HSP) 90 regulates client oncoprotein maturation. The chaperone function of HSP90 is blocked by 17-N-allylamino-17-demethoxygeldanamycin (17-AAG), although it results in transcription and translation of antiapoptotic HSP proteins. Using three myeloma cell lines, we tested whether inhibition of transcription/translation of HSP or client proteins will enhance 17-AAG-mediated cytotoxicity. 8-Chloro-adenosine (8-Cl-Ado), currently in clinical trials, inhibits bioenergy production, mRNA transcription, and protein translation and was combined with 17-AAG. 17-AAG treatment resulted in HSP transcript and protein level elevation. In the combination, 8-Cl-Ado did not abrogate HSP mRNA and protein induction. HSP90 requires ATP to stabilize client proteins; hence, expression of signal transducer and activator of transcription 3 (STAT3), Raf-1, and Akt was analyzed. 17-AAG alone resulted in <10% change in STAT3, Raf-1, and Akt protein levels, whereas no change was observed for 4E-BP1. In contrast, the combination treatment resulted in a >50% decrease in client protein levels and marked hypophosphorylation of 4E-BP1. 8-Cl-Ado alone resulted in a <30% decrease of client proteins and 4E-BP1 hypophosphorylation. 8-Cl-Ado combined with 17-AAG resulted in more than additive cytotoxicity. In conclusion, 8-Cl-Ado, which targets transcription, translation, and cellular bioenergy, enhanced 17-AAG-mediated cytotoxicity in myeloma cells.
8-Chloro-adenosine inhibits growth at least partly by interfering with actin polymerization in cultured human lung cancer cells.[Pubmed:16844099]
Biochem Pharmacol. 2006 Aug 28;72(5):541-50.
A key feature of actin is its ability to bind and hydrolyze ATP. 8-Chloro-adenosine (8-Cl-Ado), which can be phosphorylated to the moiety of 8-Cl-ATP in living cells, inhibits tumor cell proliferation. Therefore we tested the hypothesis that 8-Cl-Ado can interfere with the dynamic state of actin polymerization. We found that 8-Cl-Ado inhibited the growth of human lung cancer cell line A549 and H1299 in culture, and arrested the target cells in G2/M phase evidenced by fluorescence-activated cell sorting (FACS). Immunocytochemistry showed that the normal organization of microfilaments was disrupted in 8-Cl-Ado-exposed cells, which is accompanied by the decrease of cell size and the alteration of cell shape, and by aberrant mitosis and apoptosis in targeted cells. Furthermore, in vitro light scattering assays revealed that 8-Cl-ATP could directly inhibit the transition of G-actin to F-actin. DNase I inhibition assays showed that the G/F-actin ratio, a surrogate marker of actin polymerization status in living cells, was significantly increased in 8-Cl-Ado-exposed A549 and H1299 cells, compared to the G/F-actin ratio in unexposed cells. Taken together, these results indicate that 8-Cl-Ado exposure can alter the dynamic properties of actin polymerization, disrupt the dynamic instability or the rearrangement ability of actin filaments. Therefore, our data suggest that 8-Cl-Ado may exert its cytotoxicity at least partly by interfering with the dynamic instability of microfilaments, which may correlate with its inhibitory effects on cell proliferation and cell death.
Exposure of human lung cancer cells to 8-chloro-adenosine induces G2/M arrest and mitotic catastrophe.[Pubmed:15720807]
Neoplasia. 2004 Nov-Dec;6(6):802-12.
8-Chloro-adenosine (8-Cl-Ado) is a potent chemotherapeutic agent whose cytotoxicity in a variety of tumor cell lines has been widely investigated. However, the molecular mechanisms are uncertain. In this study, we found that exposure of human lung cancer cell lines A549 (p53-wt) and H1299 (p53-depleted) to 8-Cl-Ado induced cell arrest in the G2/M phase, which was accompanied by accumulation of binucleated and polymorphonucleated cells resulting from aberrant mitosis and failed cytokinesis. Western blotting showed the loss of phosphorylated forms of Cdc2 and Cdc25C that allowed progression into mitosis. Furthermore, the increase in Ser10-phosphorylated histone H3-positive cells revealed by fluorescence-activated cell sorting suggested that the agent-targeted cells were able to exit the G2 phase and enter the M phase. Immunocytochemistry showed that microtubule and microfilament arrays were changed in exposed cells, indicating that the dynamic instability of microtubules and microfilaments was lost, which may correlate with mitotic dividing failure. Aberrant mitosis resulted in mitotic catastrophe followed by varying degrees of apoptosis, depending on the cell lines. Thus, 8-Cl-Ado appears to exert its cytotoxicity toward cells in culture by inducing mitotic catastrophe.


